Request Demo
Knowledge Base
If the protocol changes during the clinical trial, what do the subjects need to do?
6 December 2023
2 min read
The subjects should be informed about any changes in the protocol and asked for their consent before proceeding with the new procedures. Possible consequences of not informing subjects about protocol changes include a violation of ethical principles and a lack of transparency in the study results. Additionally, if the changes are significant or involve invasive procedures, it may lead to subject withdrawal from the study or loss of trust in the research institution.
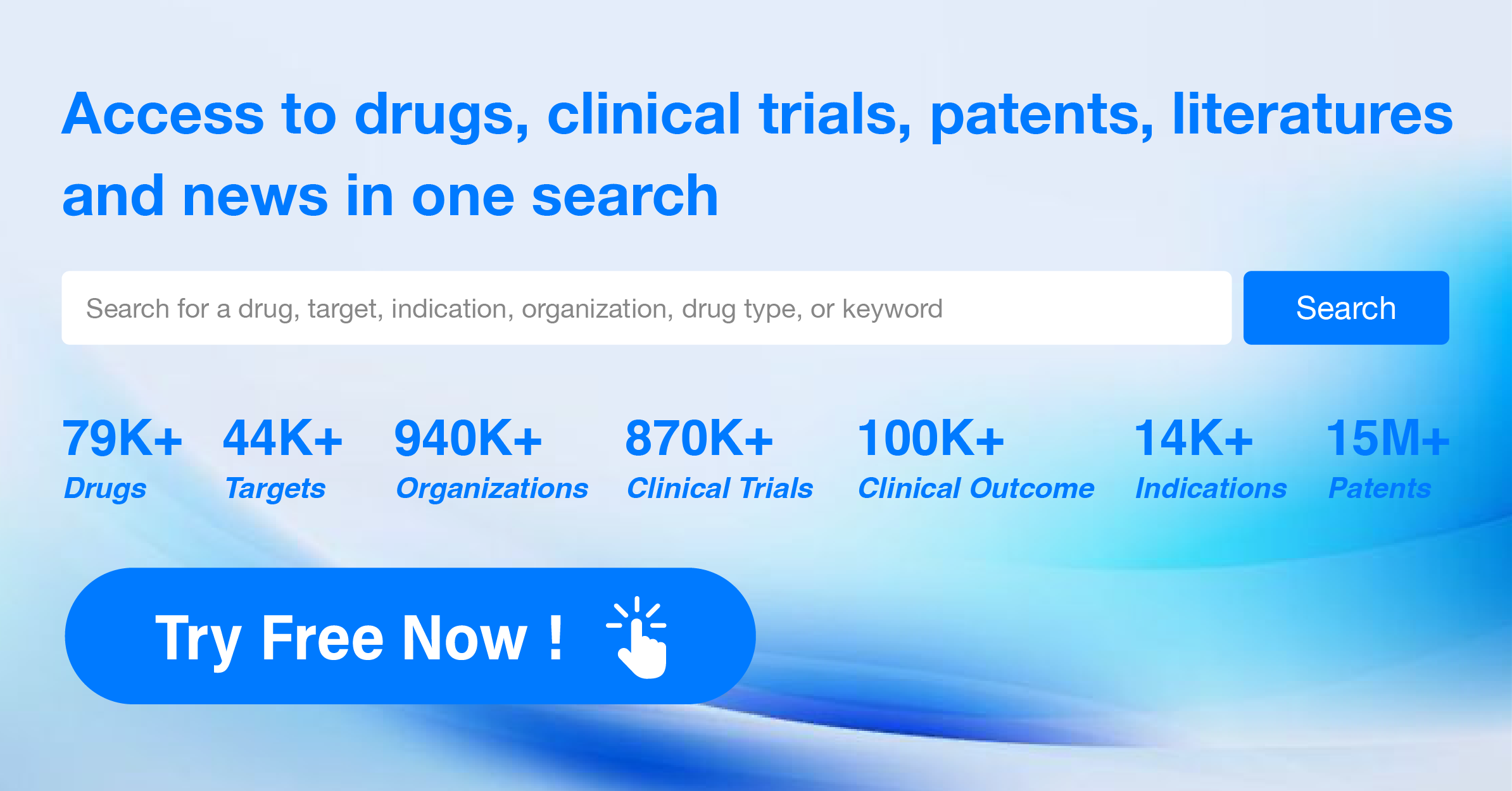
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Keep Reading
Back to all blogs →Clinical Progress
4 min read
Exploring RBN-2397's R&D successes and its clinical results at the 2023 AACR
6 December 2023
On 14 Apr 2023, the first-in-class first-in-human phase 1 trial and translational study of the mono (ADP-ribose) polymerase-7 (PARP7) inhibitor RBN-2397 was reported at the AACR Congress.
"What" Series
2 min read
What are the advantages of eCRF over CRF?
6 December 2023
The advantages of eCRF over CRF include:Digital format,Security,Transportation,Ease of use,Flexibility.
Latest Hotspot
3 min read
SpringWorks Therapeutics announced FDA approval of OGSIVEO™ (nirogacestat) as the first treatment for adult Desmoid tumor patients
6 December 2023
SpringWorks Therapeutics, Inc., a biopharma company focused on severe rare diseases and cancer, has announced FDA approval for OGSIVEO™ (nirogacestat), an oral gamma secretase inhibitor, to treat progressive desmoid tumors in adults requiring systemic therapy.
Knowledge Base
2 min read
What are the inclusion criteria for priority review?
5 December 2023
The FDA's priority review process is designed to expedite the development and review of new drugs.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.