Exploring the Latest CSF-1R inhibitor Deal by Abbisko Therapeutics: A Guide to Rapidly Accessing Transaction Insights
On December 4, 2023, Abbisko Therapeutics Co., Ltd. announced that it has entered into an exclusive license agreement with Merck, Germany, for its proprietary CSF-1R small molecule inhibitor Pimicotinib (ABSK021). Under the terms of the agreement, Merck will be granted a license to commercialize Pimicotinib for all indications in the Greater China region (Mainland China, Hong Kong, Macau and Taiwan), and Abbisko will retain the exclusive right to develop Pimicotinib within the licensed territory. In addition, Merck may exercise an option, subject to the fulfillment of the exercise conditions and the payment of additional consideration, to obtain worldwide commercialization rights for Pimicotinib, as well as the right to co-develop other indications for Pimicotinib under certain conditions. Under the terms of the agreement, Abbisko will receive a one-time, non-refundable down payment of $70 million, with additional royalties if Merck exercises its global commercialization option; together with the R&D milestone payments and sales milestone payments, these potential payments could total as much as $605.5 million, in addition to the double-digit percentage sales commissions that Merck will pay to Abbisko.
About Pimicotinib
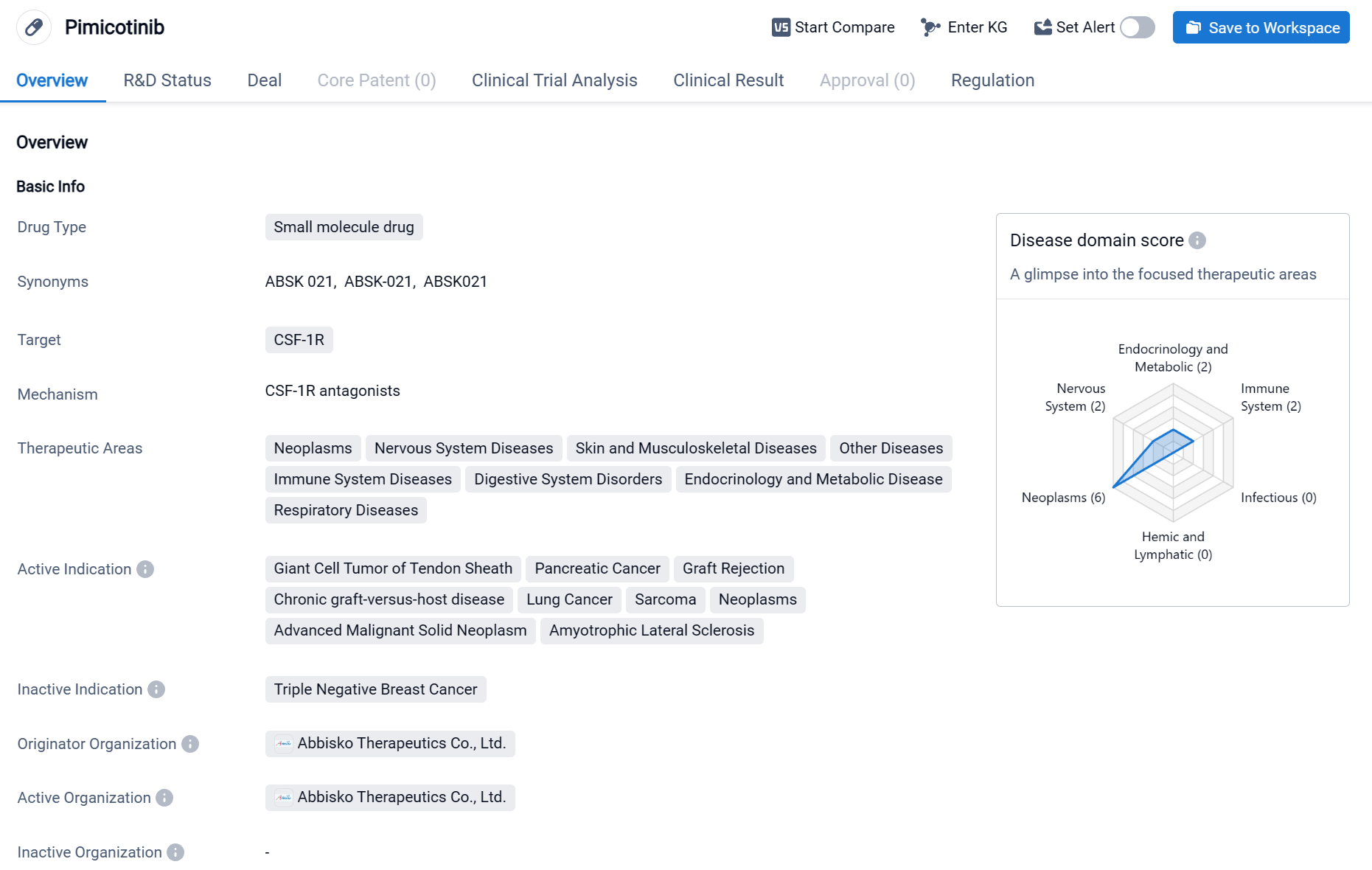
Pimicotinib is a small molecule drug that targets CSF-1R and is being developed by Abbisko Therapeutics Co., Ltd. It is currently in Phase 3 of clinical trials globally. The drug has shown potential therapeutic benefits in various therapeutic areas, including neoplasms, nervous system diseases, skin and musculoskeletal diseases, immune system diseases, digestive system disorders, endocrinology and metabolic diseases, and respiratory diseases. Click the image below to directly embark on the exploration journey with the PIMICOTINIB!
In July 2022 and January 2023, the Center for Drug Evaluation (CDE) and the United States Food and Drug Administration (FDA) respectively granted Breakthrough Therapy Designation (BTD) for pimicotinib for the treatment of tenosynovial giant cell tumor (TGCT), while the European Medicines Agency (EMA) granted it the PRIME (Priority Medicines) designation for the treatment of TGCT. This drug has completed Phase Ia dose-escalation trials in the United States and is currently conducting Phase Ib multi-cohort expansion studies in China, the US, and Europe, as well as international multi-center Phase III registration clinical trials.
About Abbisko Therapeutics Co., Ltd.
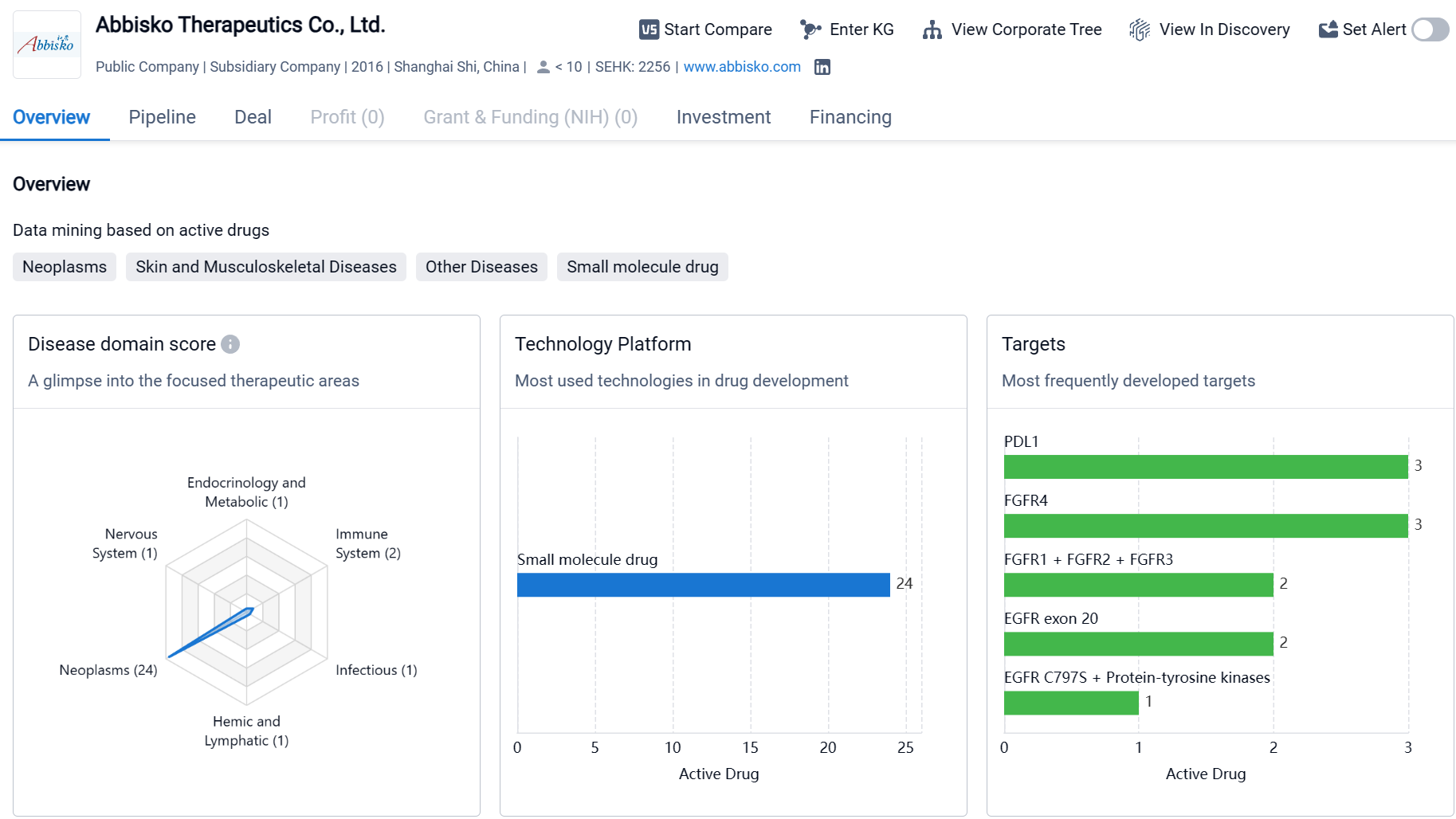
Abbisko Therapeutics Co., Ltd. is a biopharmaceutical company based in Shanghai Shi, China, with a strong focus on oncology research and development. The company has developed drugs in various therapeutic areas, with a particular emphasis on neoplasms, respiratory diseases, and digestive system disorders. Abbisko Therapeutics has also identified several molecular targets for drug development, including PDL1, FGFR1 + FGFR2 + FGFR3, and EGFR exon 20. The company has a diverse pipeline of drugs at different stages of development, with a significant number of drugs in the preclinical stage and several in clinical trials. However, as of the given date, Abbisko Therapeutics does not have any drugs that have received regulatory approval.
Currently, Abbisko Therapeutics has a product pipeline consisting of 15 candidate drugs, comprehensively covering the fields of precision oncology and cancer immunotherapy. Of these, there are eight in the clinical stage pipeline and seven in the preclinical pipeline.
How to get the latest progress on drug deals?
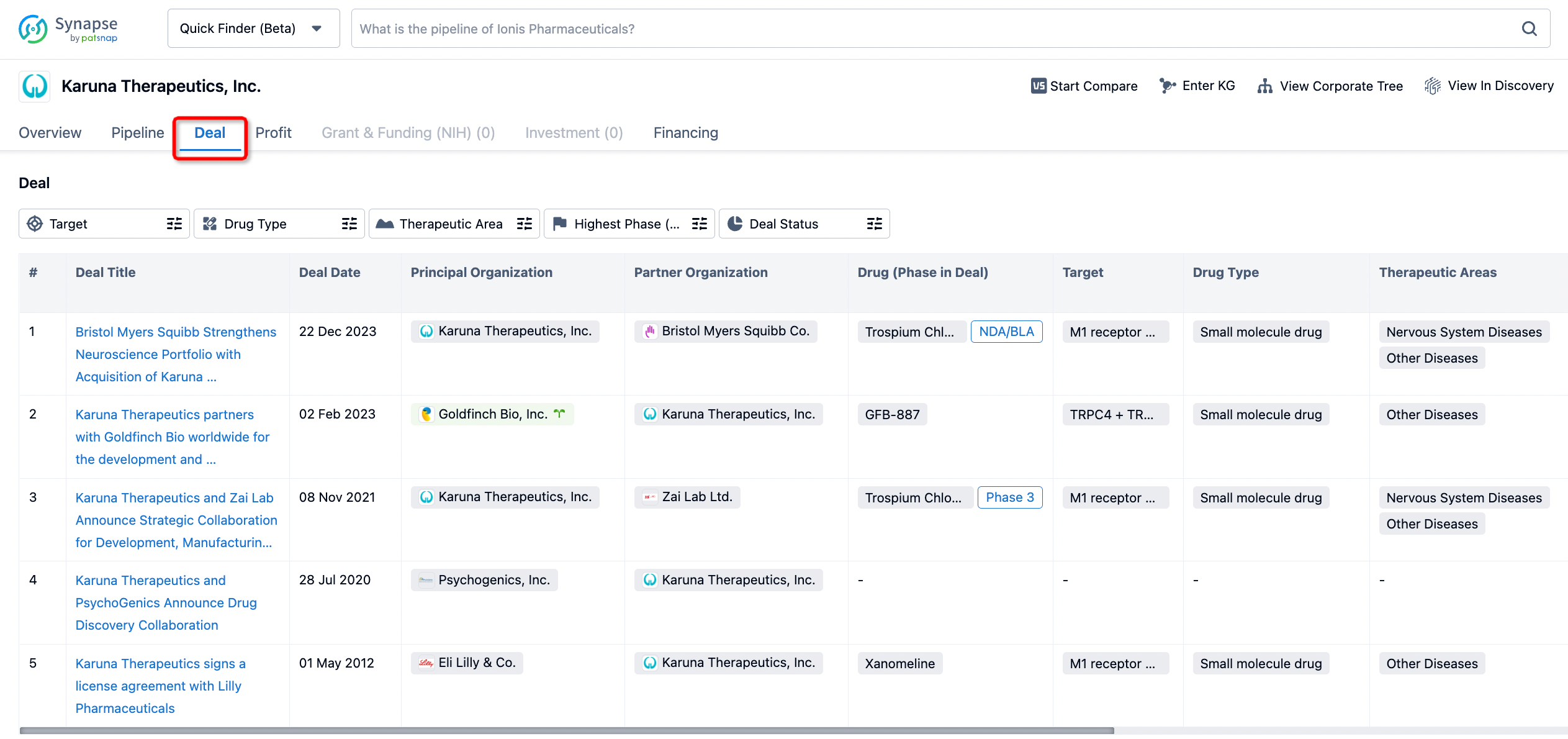
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
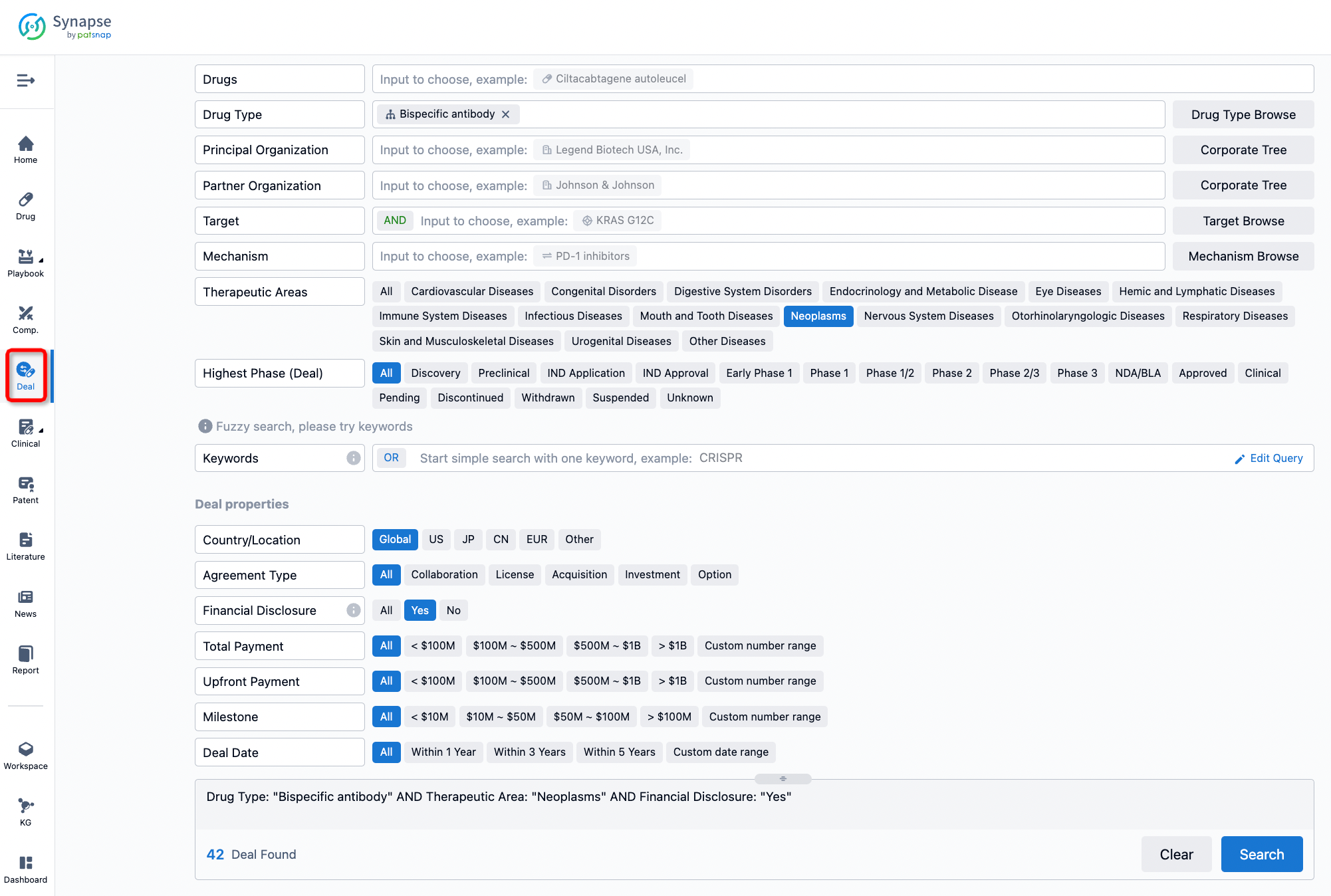
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
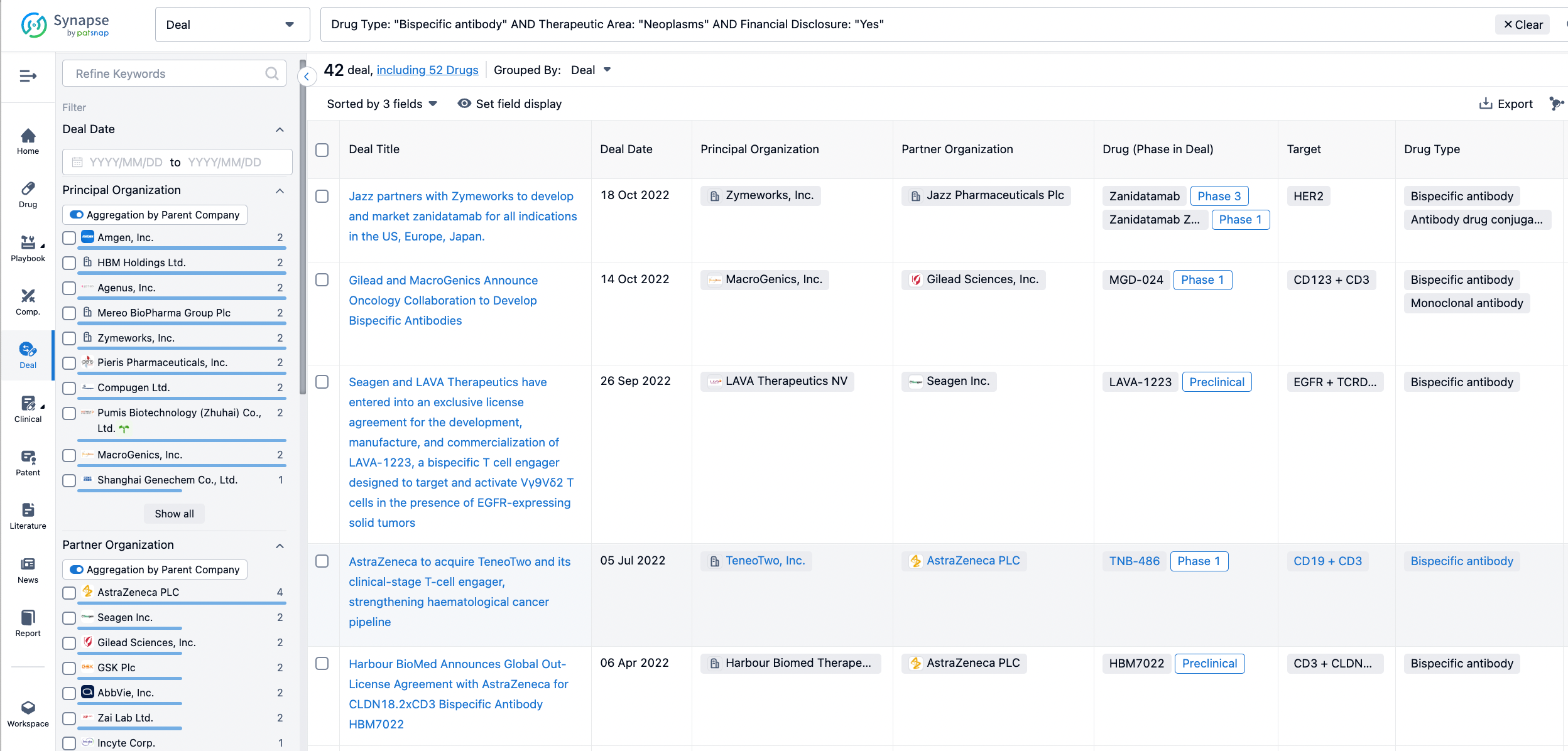
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
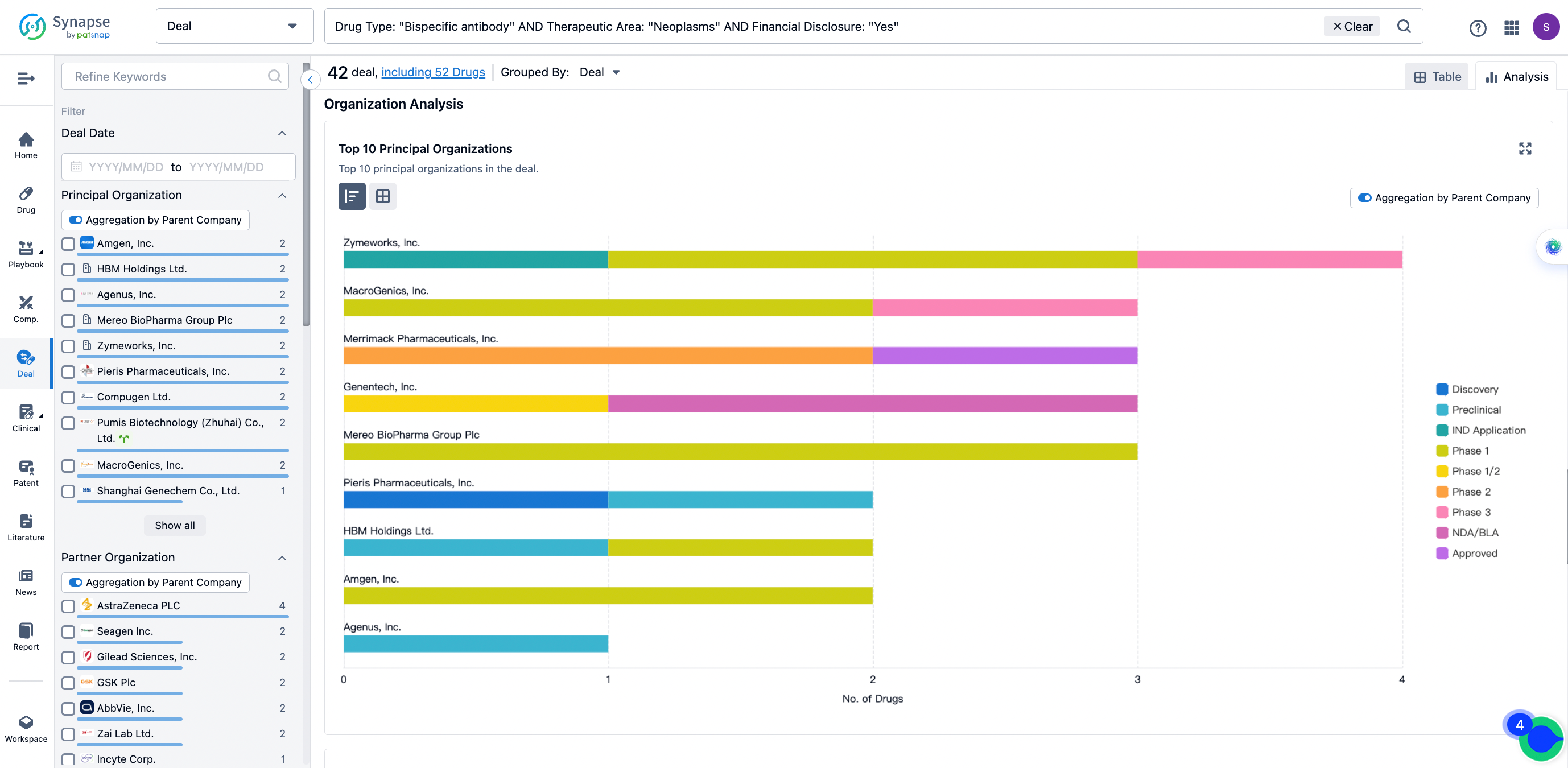
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
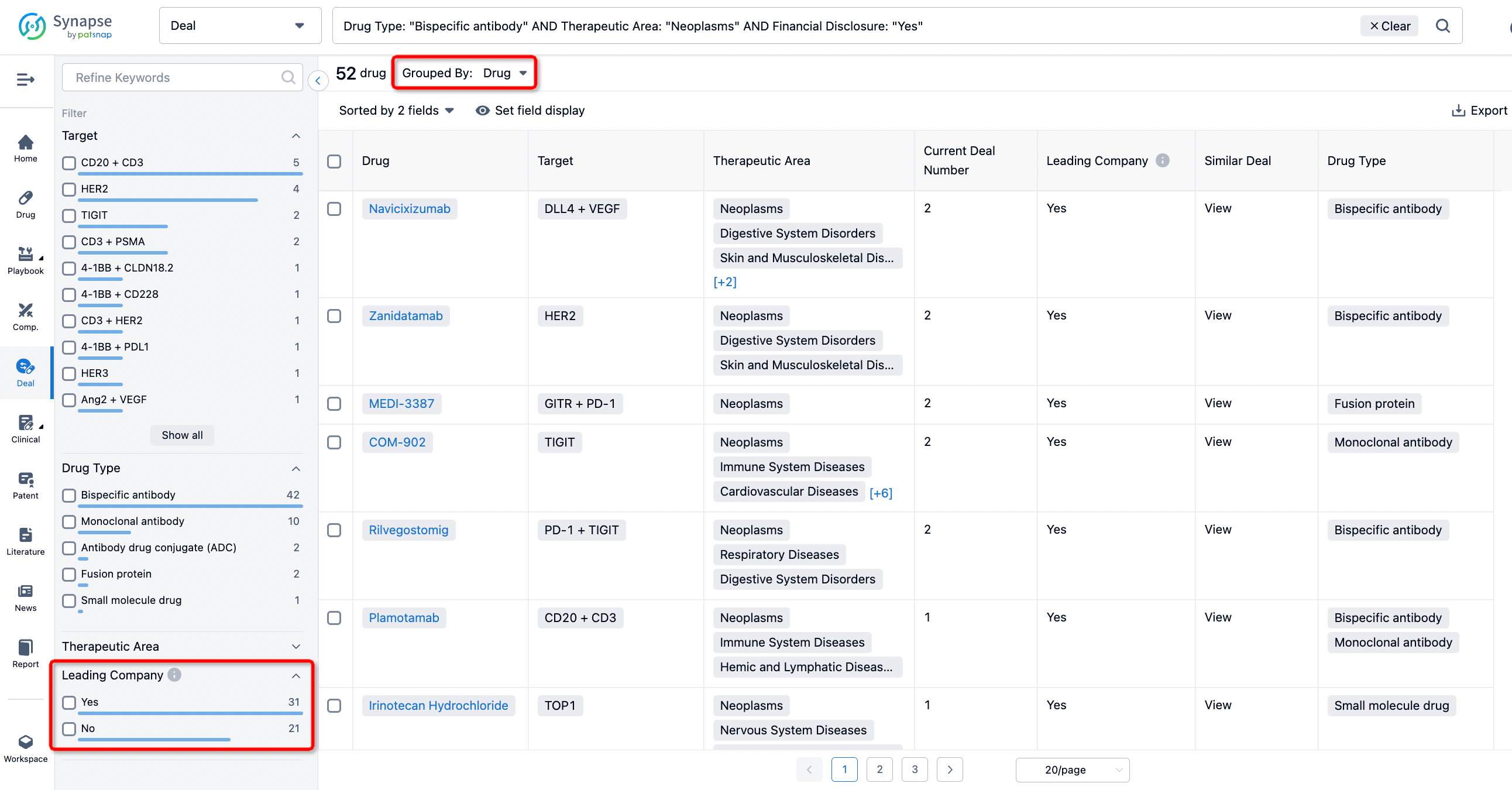
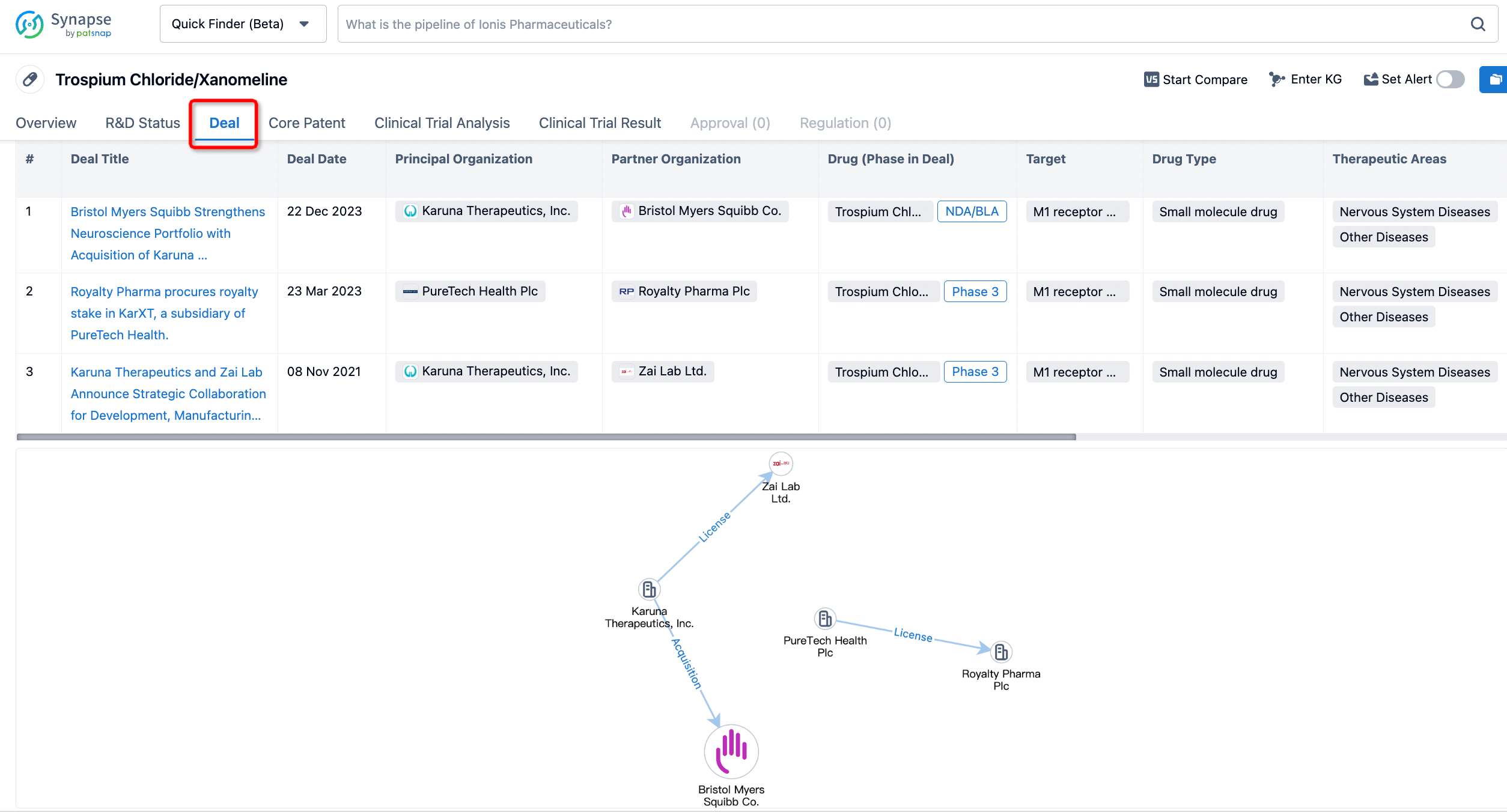
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!