Innovent announced Mazdutide's successful early Phase 3 trial in overweight and obese Chinese adults
Innovent Biologics, Inc., an esteemed global biopharmaceutical firm engaged in the creation, production, and marketing of premium medical treatments targeting cancer, autoimmune disorders, cardiovascular and metabolic diseases, ophthalmologic conditions, and other significant health issues, has declared the successful completion of the inaugural Phase 3 clinical study for mazdutide in China. Mazdutide is a dual agonist that targets both the glucagon-like peptide-1 receptor (GLP-1R) and the glucagon receptor (GCGR). The study, which involved Chinese participants dealing with excess weight or obesity, achieved its prime objective and reached all the crucial secondary objectives.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Innovent is gearing up to file the inaugural NDA to the Center for Drug Evaluation under the National Medical Products Administration of China for mazdutide, targeting weight control initiatives shortly.
GLORY-1 encompasses a large-scale, placebo-controlled, randomized, double-blind Phase 3 clinical study assessing mazdutide's effectiveness and tolerability in Chinese adults grappling with obesity or excess weight. Throughout the span of 48 weeks in the double-blind testing phase, a cohort of 610 individuals was assigned to a regimen involving either a 4 mg or 6 mg dose of mazdutide or a placebo.
The introduction of mazdutide marks the first revolutionary GLP-1R/GCGR dual agonist to achieve success in Phase 3 clinical trials. GLORY-1, as the pioneering registrational trial dedicated to weight regulation, not only reinforces the therapeutic performance and safety profile of mazdutide across a broad demographic but also furnishes robust clinical proof for the sustained pharmaceutic treatment of weight issues tailored specifically to the overweight or obese Chinese demographic.
Dr. Lei Qian, Innovent's Clinical Development VP, expressed, "Mazdutide signifies an avant-garde class of agents designed for weight reduction, distinguished as an innovative GLP-1R/GCGR dual agonist. It boasts extensive clinical investigations involving a participant pool exceeding a thousand from China. Our team is meticulously finalizing the findings and anticipates lodging mazdutide's maiden NDA for weight regulation prospectively. Our mission is to make available a dependable and potent anti-obesity treatment for the Chinese demographic afflicted with obesity."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
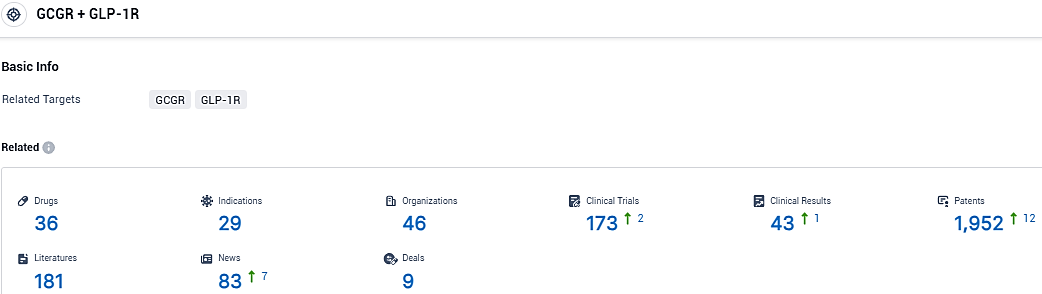
According to the data provided by the Synapse Database, As of January 13, 2024, there are 36 investigational drugs for the GCGR and GLP-1R tagets, including 29 indications, 46 R&D institutions involved, with related clinical trials reaching 173, and as many as 1952 patents.
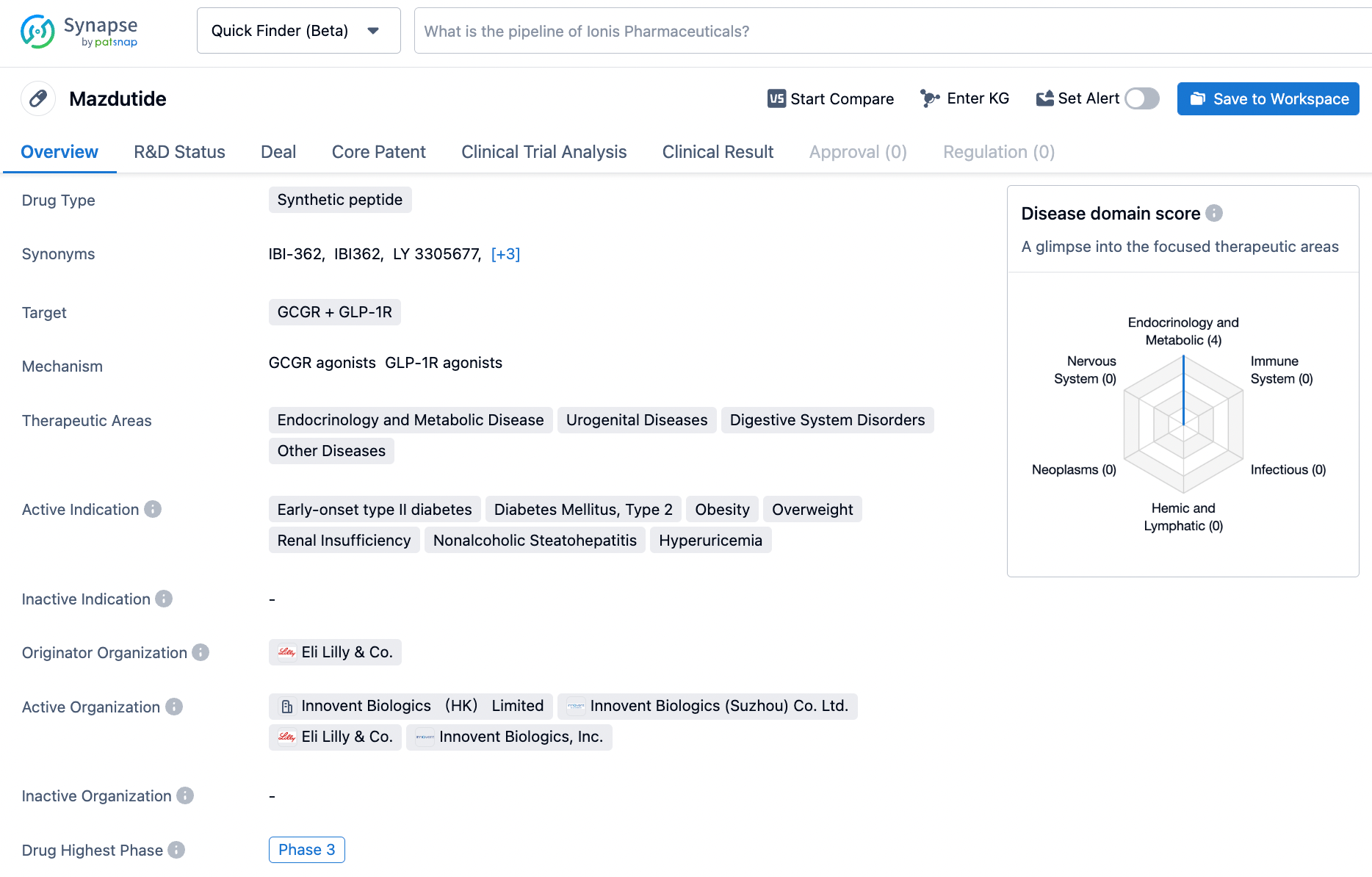
Mazdutide has potential applications in various therapeutic areas, including endocrinology and metabolic diseases, urogenital diseases, digestive system disorders, and other diseases. The drug shows promise in addressing conditions such as early-onset type II diabetes, diabetes mellitus type 2, obesity, overweight, renal insufficiency, nonalcoholic steatohepatitis, and hyperuricemia. Further research and clinical trials are needed to fully understand the efficacy and safety profile of Mazdutide in these indications.