Immutep Reports Initial Dosage in Phase I Trial of IMP761, an Innovative LAG-3 Agonist Antibody
Immutep Limited, a biotechnology firm in the clinical-stage focused on developing new LAG-3 immunotherapies for cancer and autoimmune conditions, has announced the successful dosing of the initial participant in the first-in-human Phase I trial of IMP761.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 This pioneering LAG-3 agonist antibody aims to rebalance the immune system by enhancing the inhibitory role of LAG-3, thereby silencing dysregulated self-antigen-specific memory T cells responsible for numerous autoimmune disorders.
This pioneering LAG-3 agonist antibody aims to rebalance the immune system by enhancing the inhibitory role of LAG-3, thereby silencing dysregulated self-antigen-specific memory T cells responsible for numerous autoimmune disorders.
A Phase I study, using single and multiple ascending doses, along with a placebo-controlled, double-blind setup, is being executed by the Centre for Human Drug Research (CHDR)—an esteemed institution in Leiden, the Netherlands known for leading-edge early-phase clinical drug investigations. The trial seeks to recruit 49 healthy participants to evaluate safety, pharmacokinetics, and pharmacodynamics.
CHDR will utilize its exclusive keyhole limpet haemocyanin challenge model, which facilitates early pharmacodynamic activity assessment of IMP761. Immutep expects to obtain initial safety data from this Phase I study by the end of this year, with further analysis of PK/PD relationships projected in the first half of 2025.
LAG-3 is recognized as a promising checkpoint for agonist LAG-3 immunotherapy for treating autoimmune conditions such as rheumatoid arthritis, Type 1 diabetes, and multiple sclerosis. Preclinical experiments have shown that IMP761 significantly reduces inflammatory cytokines and effectively suppresses antigen-specific T cell-driven immune reactions.
IMP761, recognized as a groundbreaking immunosuppressive LAG-3 agonist antibody, holds the potential to tackle the underlying causes of many autoimmune conditions by selectively silencing autoimmune memory T cells that gather at disease sites, thereby restoring immune system equilibrium. Studies published in the Journal of Immunology highlight that IMP761 successfully inhibits peptide-induced T cell proliferation, the activation of human primary T cells, and an antigen-specific delayed-type hypersensitivity reaction in both in vitro and in vivo settings.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
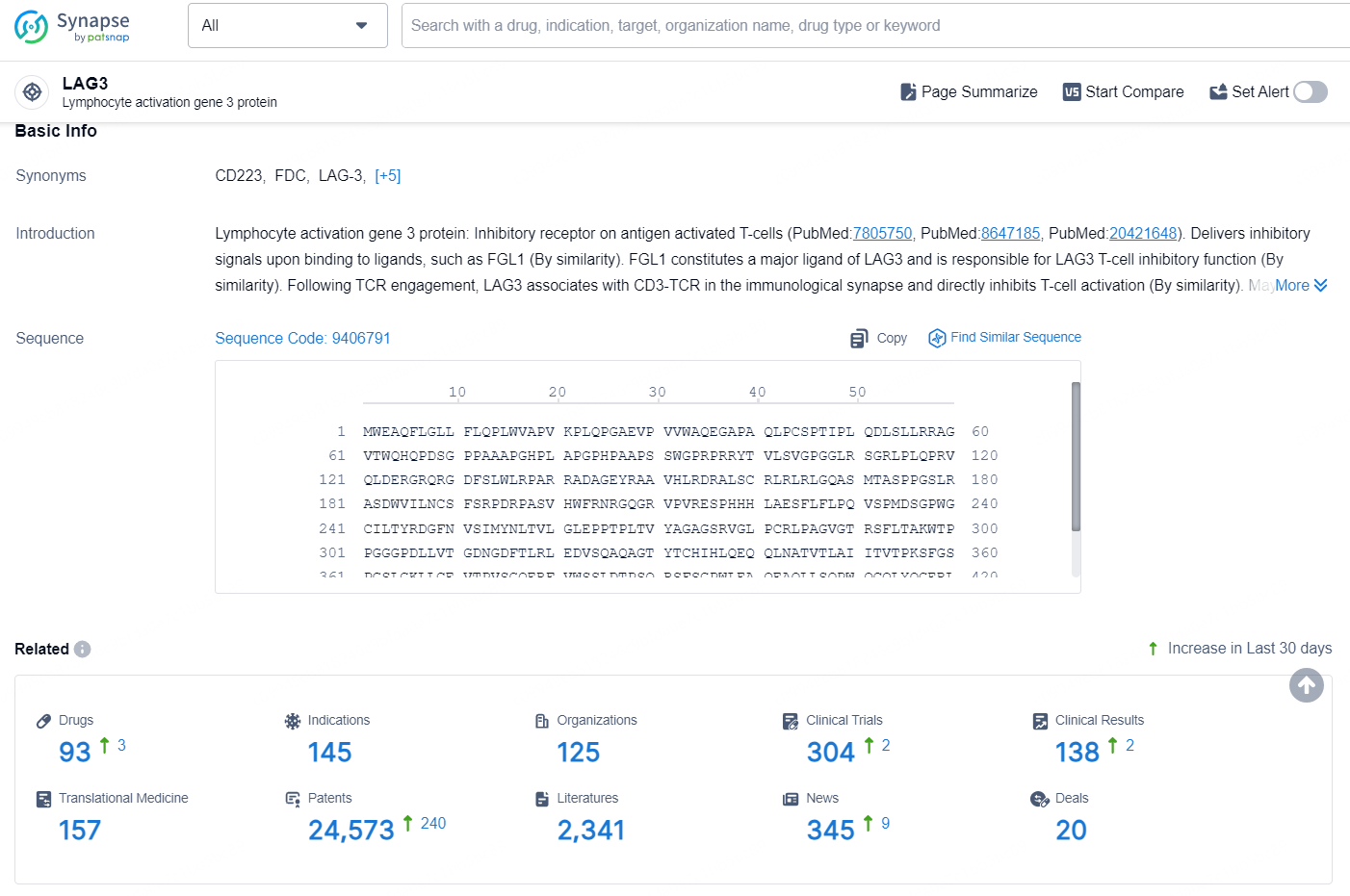
According to the data provided by the Synapse Database, As of August 20, 2024, there are 93 investigational drugs for the LAG-3 targets, including 145 indications, 125 R&D institutions involved, with related clinical trials reaching 304, and as many as 24573 patents.
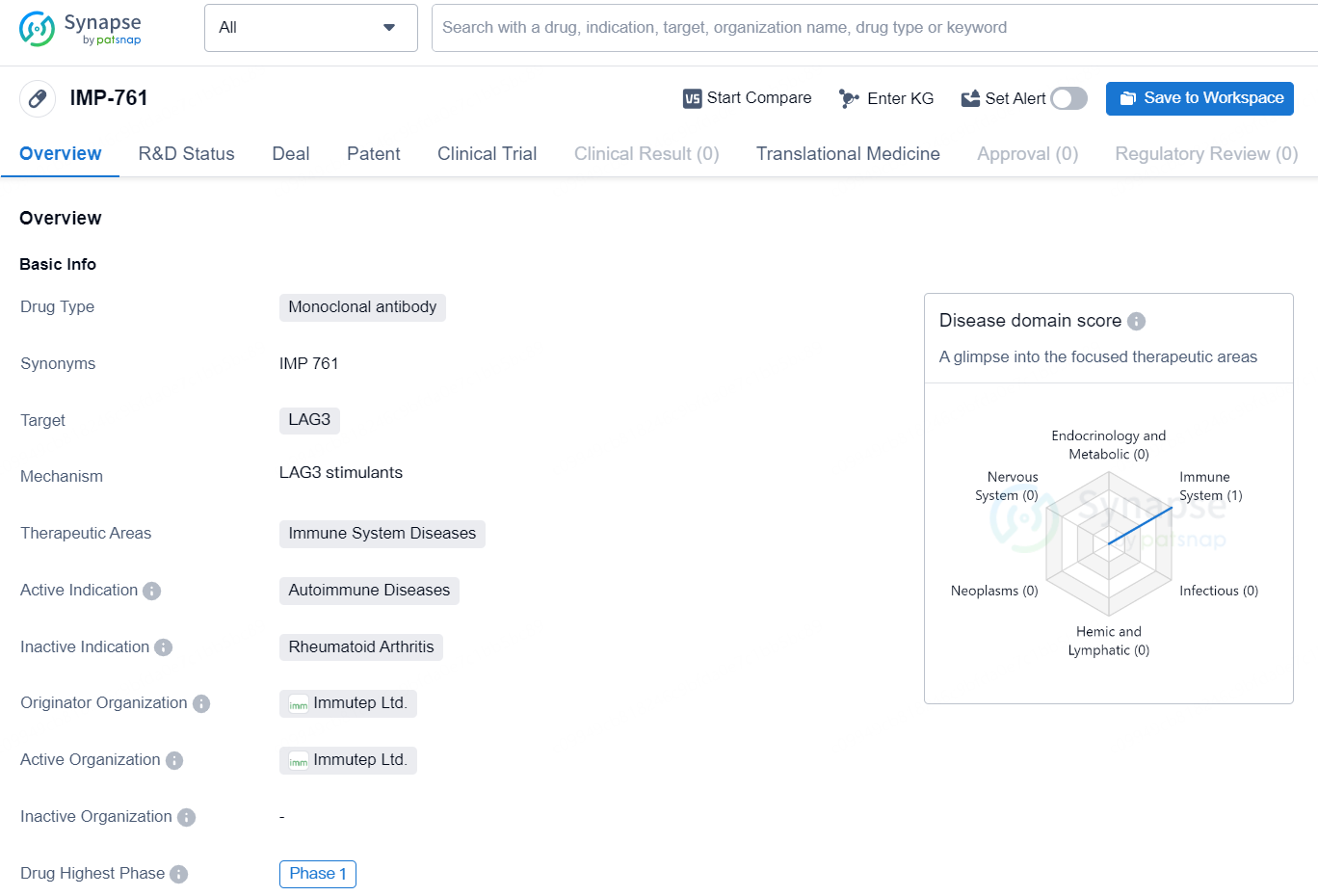
IMP-761 represents a novel approach to treating autoimmune diseases by targeting LAG3 with a monoclonomic antibody. As the drug progresses through clinical development, further data will be needed to determine its efficacy and safety for patients with autoimmune conditions.