Fate Therapeutics Presents Preclinical Data on MICA/B-targeted CAR T-cell Therapy FT836 at 2024 SITC
Fate Therapeutics, Inc. (NASDAQ: FATE), a biopharmaceutical company in the clinical stage focusing on the development of innovative induced pluripotent stem cell (iPSC)-derived cellular immunotherapies for cancer and autoimmune diseases, recently shared early preclinical findings for FT836. This is a multiplex-engineered chimeric antigen receptor (CAR) T-cell product candidate that targets major histocompatibility complex (MHC) proteins A (MICA) and B (MICB). The presentation took place at the 2024 Society of Immunotherapy of Cancer (SITC) 39th Annual Meeting, which is scheduled from November 6-10, 2024, in Houston, TX.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The cell-surface proteins MICA/B are upregulated in response to cellular stress or malignant changes and can be observed in various cancer cells while showing limited presence on healthy tissues. FT836 features several advanced synthetic regulatory elements for CAR T-cell function, including the Company’s proprietary Sword & Shield technology. This technology consists of a series of genetic modifications aimed at both targeting and avoiding host alloreactive immune responses to ensure maintained functionality of ready-to-use CAR T-cell therapies without the need for conditioning chemotherapy.
"The innovative set of synthetic controls built into FT836 is developed to tackle key obstacles that have constrained CAR T-cell safety and effectiveness in solid tumors. These obstacles include on-target/off-tumor toxicity, suppression of effector cells within the tumor environment, tumor diversity, and restricted functionality over time," stated Bob Valamehr, Ph.D., President of Research & Development at Fate Therapeutics. "Our preclinical data on FT836, unveiled at SITC, demonstrate MICA/B targeting's effectiveness across multiple cancer types and suggest our advanced iPSC-derived CAR T-cell platform may offer strong and lasting anti-tumor effects without needing conditioning chemotherapy to diminish host immune cells."
Targeting MICA/B is gaining traction as a new approach focused specifically on cancer to tackle various solid tumors. Nonetheless, a frequent method of cancer resistance is through proteolytic cleavage and shedding of MICA/B at the α3 domain near the membrane, which helps cancers evade standard NKG2D-mediated recognition. FT836 is specially engineered to attach to the α3 domain, which has been demonstrated to stabilize MICA/B expression and stimulate strong tumor cell cytolysis. During today’s oral presentation at SITC called "Development of an Off-the-Shelf, MICA/B Targeting CAR T Cell to Combat Pan-tumor Escape Mechanism for Solid Tumors," Company scientists showcased that FT836 demonstrated powerful and durable anti-cancer activity in vivo across an extensive range of solid tumors. Additionally, treating tumor cells with chemotherapy or radiation therapy in vitro boosted MICA/B expression and enhanced the cytolytic effects of FT836, suggesting the potential for combining with standard treatment protocols for solid tumors.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
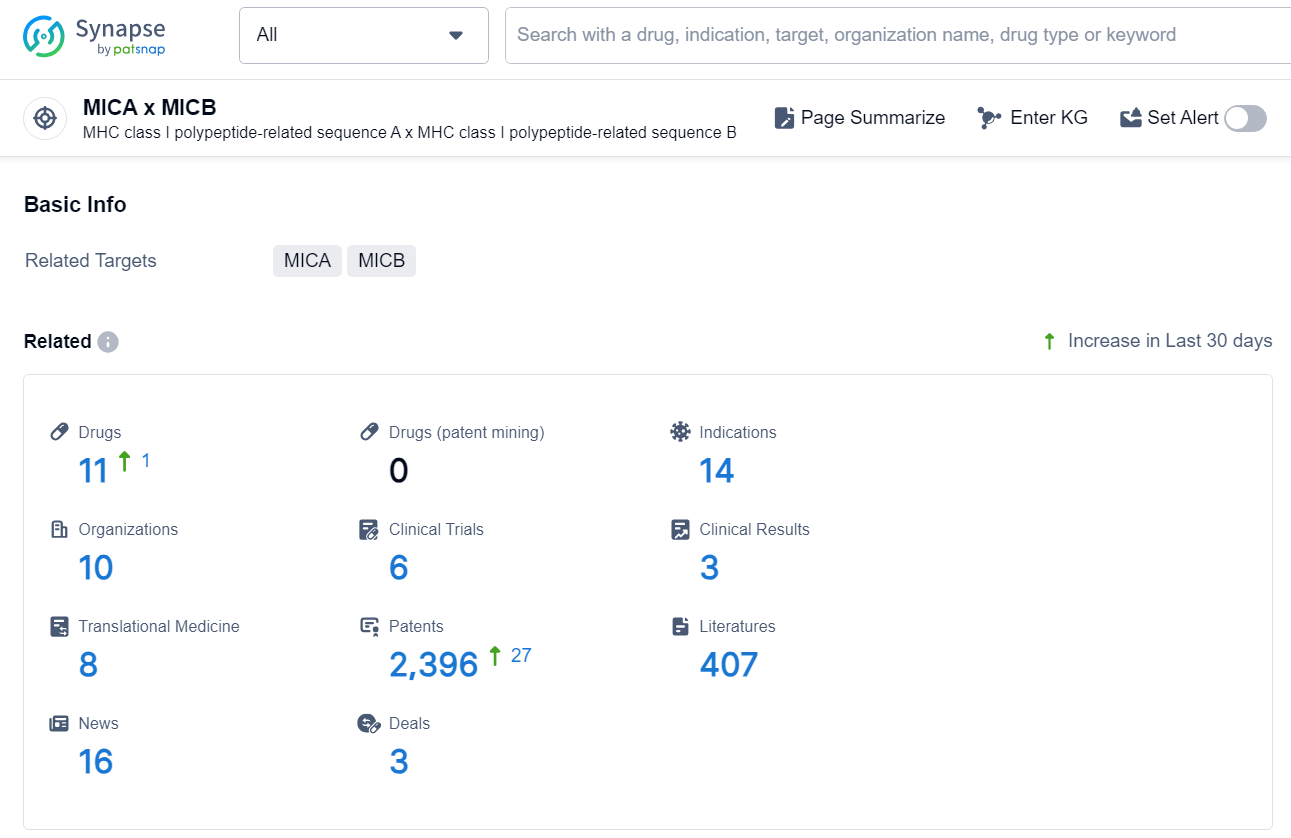
According to the data provided by the Synapse Database, As of November 13, 2024, there are 11 investigational drugs for the MICA and MICB target, including 14 indication, 10 R&D institutions involved, with related clinical trial reaching 6, and as many as 2396 patents.
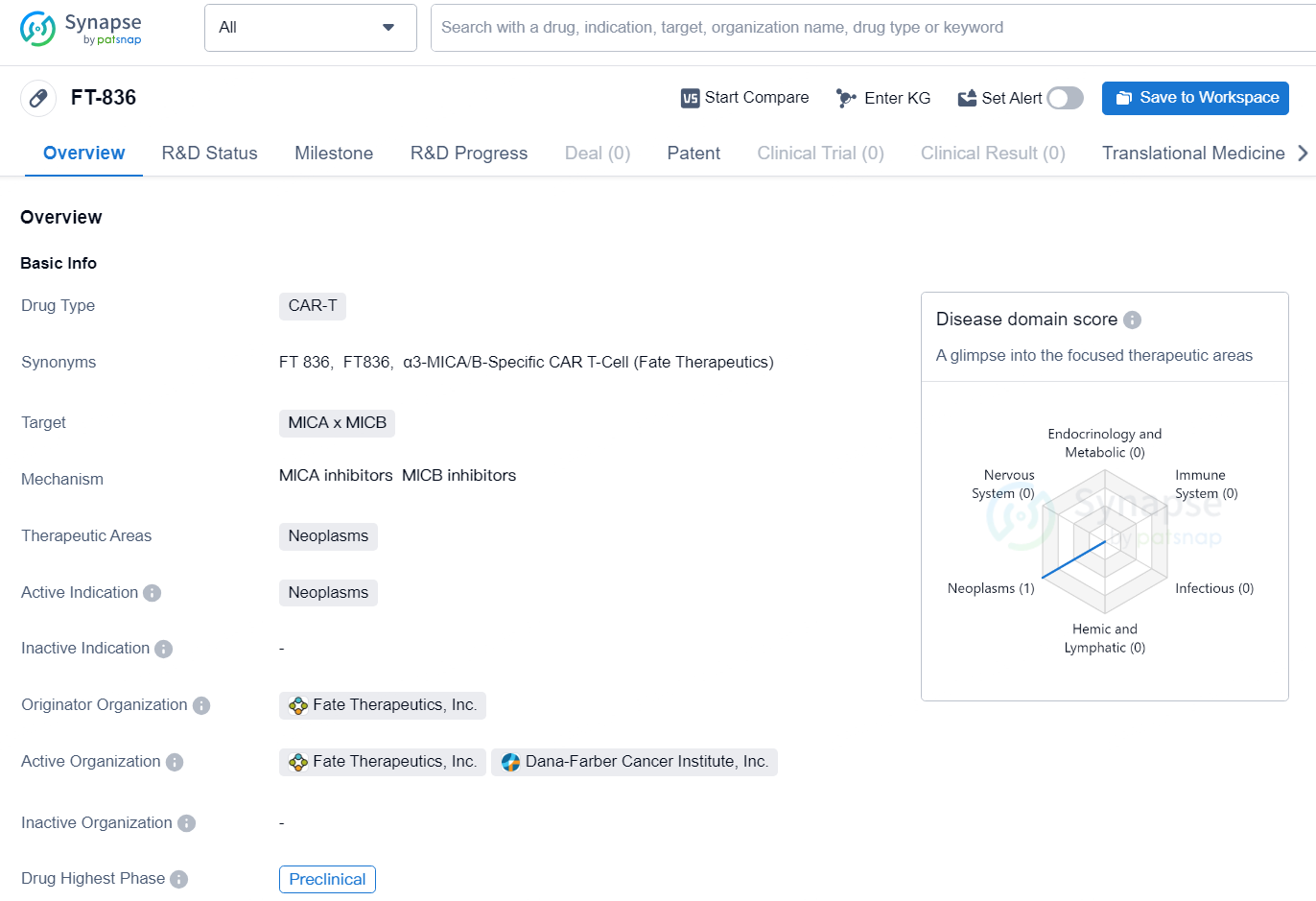
In the field of biomedicine, the drug [FT-836] is classified as a CAR-T type drug, designed to target the MICA and MICB proteins. The therapeutic areas in which this drug is intended to be used are neoplasms, and its active indication is for the treatment of neoplasms. The originator organization of this drug is Fate Therapeutics, Inc. as the pharmaceutical company responsible for its development.