InnoCare Reveals approval of the Clinical Experiment for ICP-189, Coupled with EGFR Inhibitor Furmonertinib
InnoCare Pharma, a prominent biopharmaceutical corporation specializing in cancer and autoimmune disease therapies, has revealed today that it has received IND approval to carry out a clinical study in China. This trial will examine the effects of a new SHP2 allosteric inhibitor, ICP-189 , in conjunction with furmonertinib, an EGFR inhibitor known for its high brain penetration and broad mutation-specific activity.
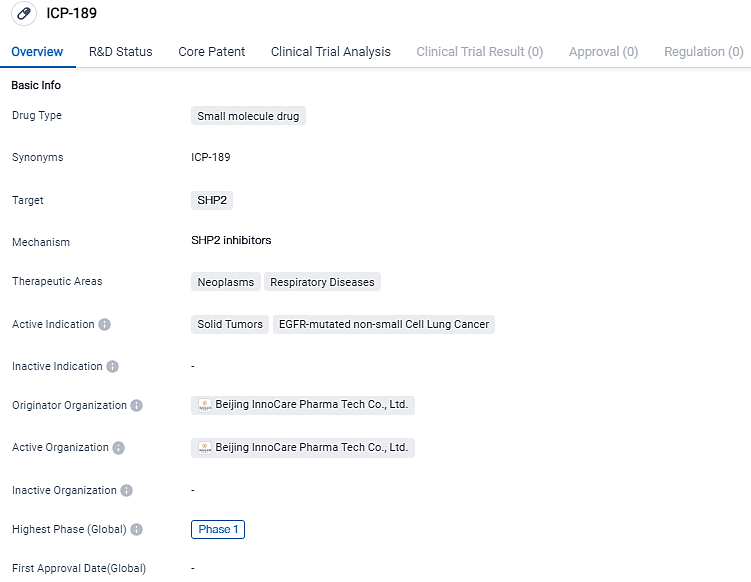
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the middle of July, a clinical development partnership was unveiled by InnoCare and ArriVent. The aim was to examine the anti-cancer properties and safety of the combination of furmonertinib and ICP-189 in people battling with advanced non-small cell lung cancer.
ArriVent is progressing with global research on furmonertinib for patients with advanced or metastatic NSCLC with EGFR or HER2 mutations. This includes those with exon 20 insertion mutations and other rare EGFR mutations. It has been given approval in China for first-line treatment for adults afflicted with locally advanced or metastatic NSCLC possessing EGFR exon 19 deletion or exon 21 substitution mutations.
A dosage escalation study revealed that the dosage has successfully been escalated to 120mg with no observed DLT, and a favorable safety and PK profile has been exhibited. Initial efficacy was seen in ICP-189 as a monotherapy. In the 20mg dosage cohort, a patient suffering from cervical cancer achieved a confirmed partial response.
InnoCare's Co-Founder, Chairwoman, and CEO, Dr. Jasmine Cui voiced that the SHP2 inhibitor is the optimal partner for combinational use with various targeted and immune-oncology therapies in the realm of solid tumors. She stated that ICP-189 has shown remarkable anti-tumor efficacy in tumor models driven by KRASG12C mutation or EGFR over-production. InnoCare plans to collaborate with ArriVent to expedite the clinical development of the ICP-189 and furmonertinib combination. This innovative treatment is intended to assist patients with advanced NSCLC at an earlier stage.
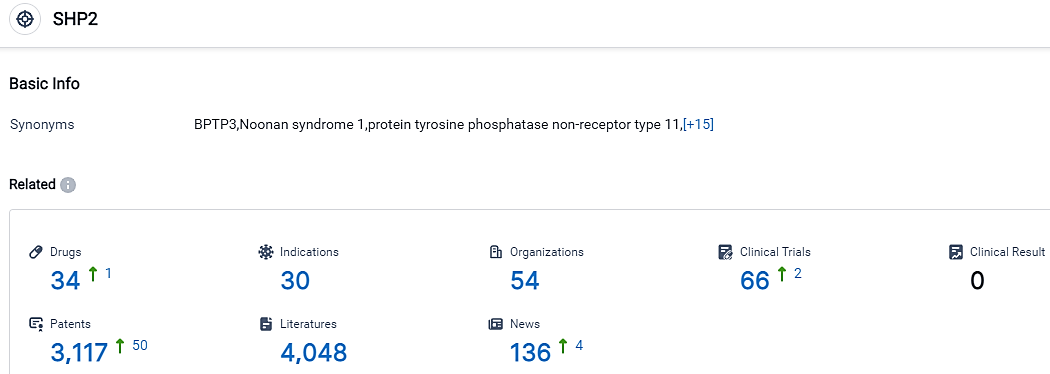
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 18, 2023, there are 34 investigational drugs for the SHP2 target, including 30 indications, 54 R&D institutions involved, with related clinical trials reaching 66,and as many as 3117 patents.
ICP-189 is a potent and selective oral allosteric inhibitor of SHP2, developed by InnoCare for the treatment of solid tumors as a single agent and/or in combination with other antitumor agents. It targets SHP2 and is indicated for the treatment of solid tumors and EGFR-mutated non-small cell lung cancer. ICP-189 shows promise in the field of biomedicine for potential therapeutic interventions in neoplasms and respiratory diseases.