Innovent Reveals Phase 3 Results of Mazdutide for Weight Control at ADA 84th Sessions
Innovent Biologics, Inc., a leading global biopharmaceutical company dedicated to the development, production, and commercialization of high-quality medications for oncology, autoimmune, cardiovascular and metabolic disorders, ophthalmology, and other significant diseases, will unveil the primary outcomes of the initial Phase 3 clinical trial of mazdutide in Chinese adults with overweight or obesity during the ADA Scientific Sessions 2024.
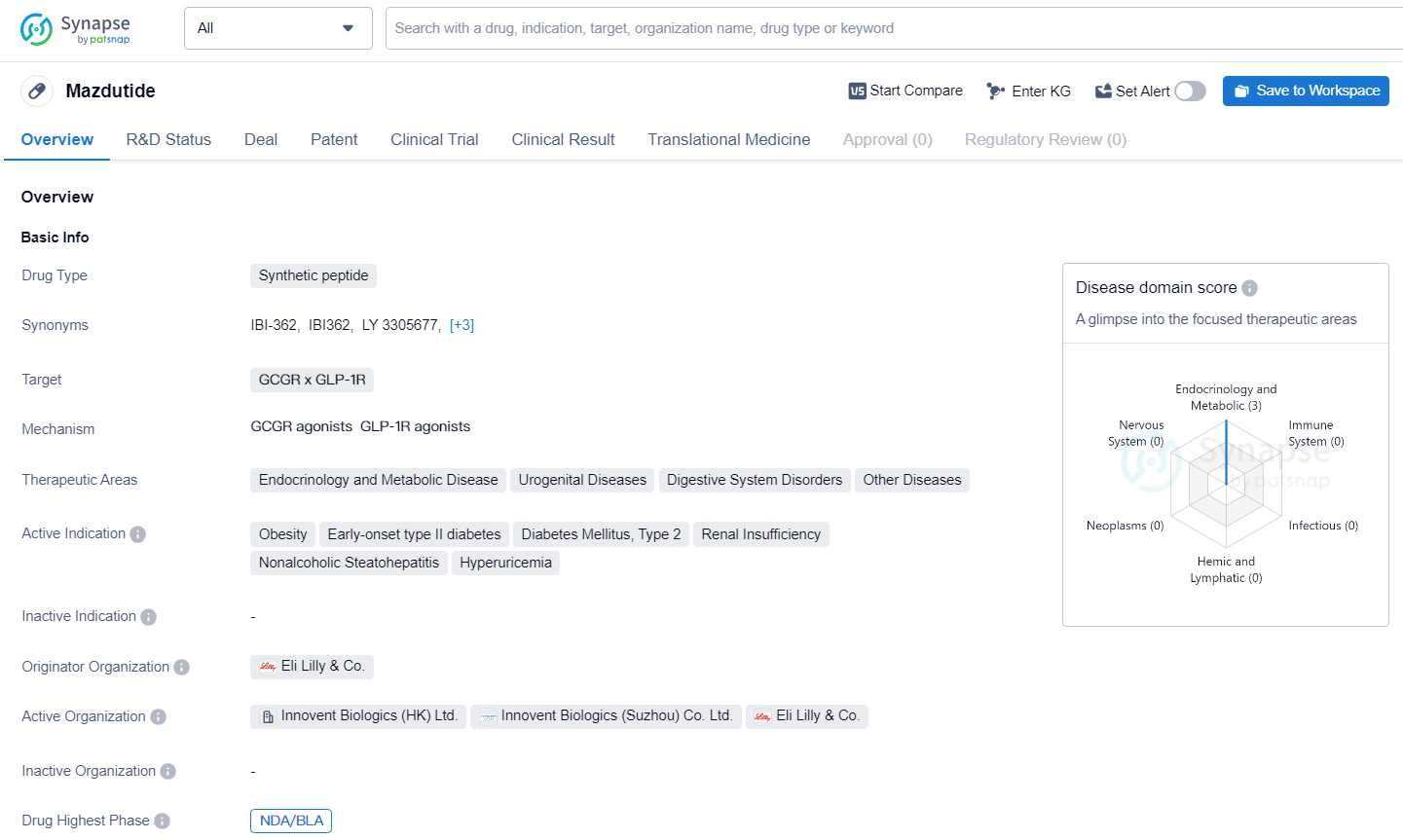
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Mazdutide acts as a dual agonist targeting both the glucagon-like peptide-1 receptor (GLP-1R) and the glucagon receptor (GCGR). Activation of GLP-1R by mazdutide helps to suppress appetite and slow gastric emptying, which contributes to weight reduction. Concurrently, by stimulating GCGR, it promotes increased energy expenditure, fatty acid oxidation, and lipolysis, along with a reduction in liver fat.
The GLORY-1 study findings revealed that mazdutide effectively induces significant weight loss in overweight and obese adults, while also lowering liver fat and several cardiometabolic risk indicators. Its maiden new drug application for chronic weight management is under evaluation by the CDE of the National Medical Products Administration. Upon approval, mazdutide is anticipated to be a safe and convenient treatment for obesity, aiding in substantial weight reduction with cardiovascular and metabolic advantages.
Detailed results and analyses from GLORY-1 are slated for publication in peer-reviewed academic journals. To date, more than 1,500 participants have been treated with mazdutide across 17 clinical trials, solidifying its clinical validation for weight management and diabetes care in China.
Throughout the 48-week treatment period, the mean heart rate changes from baseline did not exceed 5 beats/min in both the 4 mg and 6 mg mazdutide groups. Specifically, the average heart rate change at week 48 was 1.6 beats/min for both dosages. No indicators of increased cardiovascular risk were detected during the therapy.
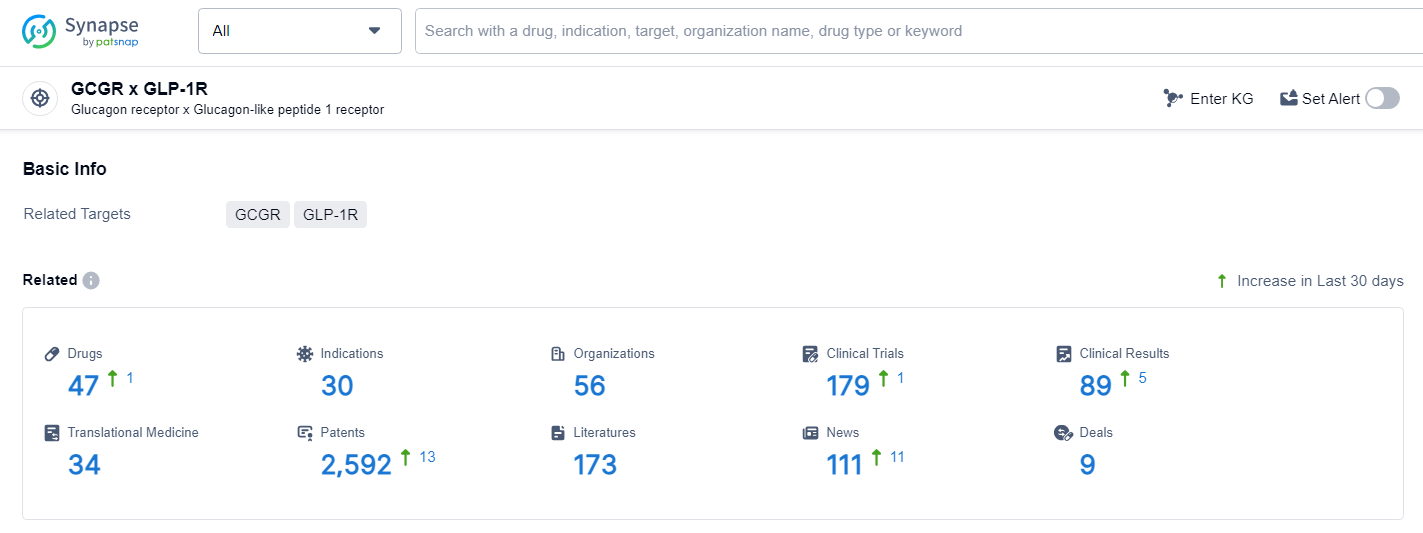
Dr. Lei Qian, Vice President of Clinical Development at Innovent, shared, “We are thrilled to showcase the GLORY-1 findings at the ADA. The study indicates that the GLP-1R/GCGR dual agonist mazdutide is a viable and well-tolerated option for helping individuals who are overweight or obese to lose weight, decrease liver fat, and mitigate other obesity-related comorbidities. Additionally, we plan to release mazdutide results for type 2 diabetes later this year. Innovent is committed to advancing our next-generation cardiovascular and metabolic therapies, aiding individuals in the pursuit of healthier lives.”👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 28, 2024, there are 47 investigational drugs for the GCGR and GLP-1R target, including 30 indications, 56 R&D institutions involved, with related clinical trials reaching 179, and as many as 2588 patents.
Mazdutide represents a significant advancement in the field of biomedicine, particularly in the treatment of endocrine and metabolic diseases. The drug's highest phase of development in both global and Chinese markets indicates a strong potential for commercialization and widespread impact on patient care. As the drug progresses through the approval process, it will be important to monitor its efficacy and safety profile in diverse patient populations to fully understand its potential benefits.