Is Sevenfact approved by the FDA?
Yes, Sevenfact is FDA approved. The U.S. Food and Drug Administration (FDA) granted approval to Sevenfact on April 1, 2020. Sevenfact, a recombinant coagulation factor VIIa, is used in the treatment and prevention of bleeding episodes in patients with Hemophilia A or B who have developed inhibitors against other clotting proteins such as Factor VIII or Factor IX.
What is Sevenfact?
Sevenfact is a brand name for a coagulation factor VIIa (recombinant)-jncw. It is designed to replicate the naturally occurring activated factor VII (factor VIIa) in the body, which helps to initiate the clotting process and control bleeding. It is indicated for use in:
- Hemophilia A or B with inhibitors
- Acquired hemophilia
- Congenital Factor VII deficiency
- Glanzmann’s thrombasthenia
Administration and Dosage
Sevenfact is administered via intravenous injection by healthcare professionals. The dosage varies based on the patient's condition and severity of the bleeding episode. The specific dosing regimen should be determined by a healthcare provider.
Warnings and Precautions
- Thrombosis: There is a risk of serious arterial and venous thrombotic events associated with the use of Sevenfact. Patients should be monitored for signs of thrombosis and activation of the coagulation system.
- Allergic Reactions: Sevenfact may cause serious allergic reactions, including anaphylaxis. Patients should seek immediate medical attention if they experience symptoms such as rash, itching, trouble breathing, or swelling of the face, hands, or mouth.
- Interactions: Sevenfact may interact with other medications, particularly those affecting blood clotting. Patients should inform their healthcare provider about all medicines they are taking.
Side Effects
Common side effects of Sevenfact include:
- Bleeding problems
- Fever
- High blood pressure
- Joint or muscle pain or stiffness
Serious side effects that require medical attention include:
- Severe headache
- Vision or speech problems
- Chest pain
- Shortness of breath
- Signs of blood clots
Conclusion
Sevenfact (coagulation factor VIIa) is an FDA-approved medication for managing bleeding episodes in patients with Hemophilia A or B who have inhibitors, as well as other related bleeding disorders. Approved on April 3, 2020, it offers a vital treatment option for patients who need an alternative to traditional clotting factor therapies. Patients receiving Sevenfact should be closely monitored by healthcare professionals due to the risk of thrombotic events and other serious side effects.
How to obtain the latest development progress of all drugs?
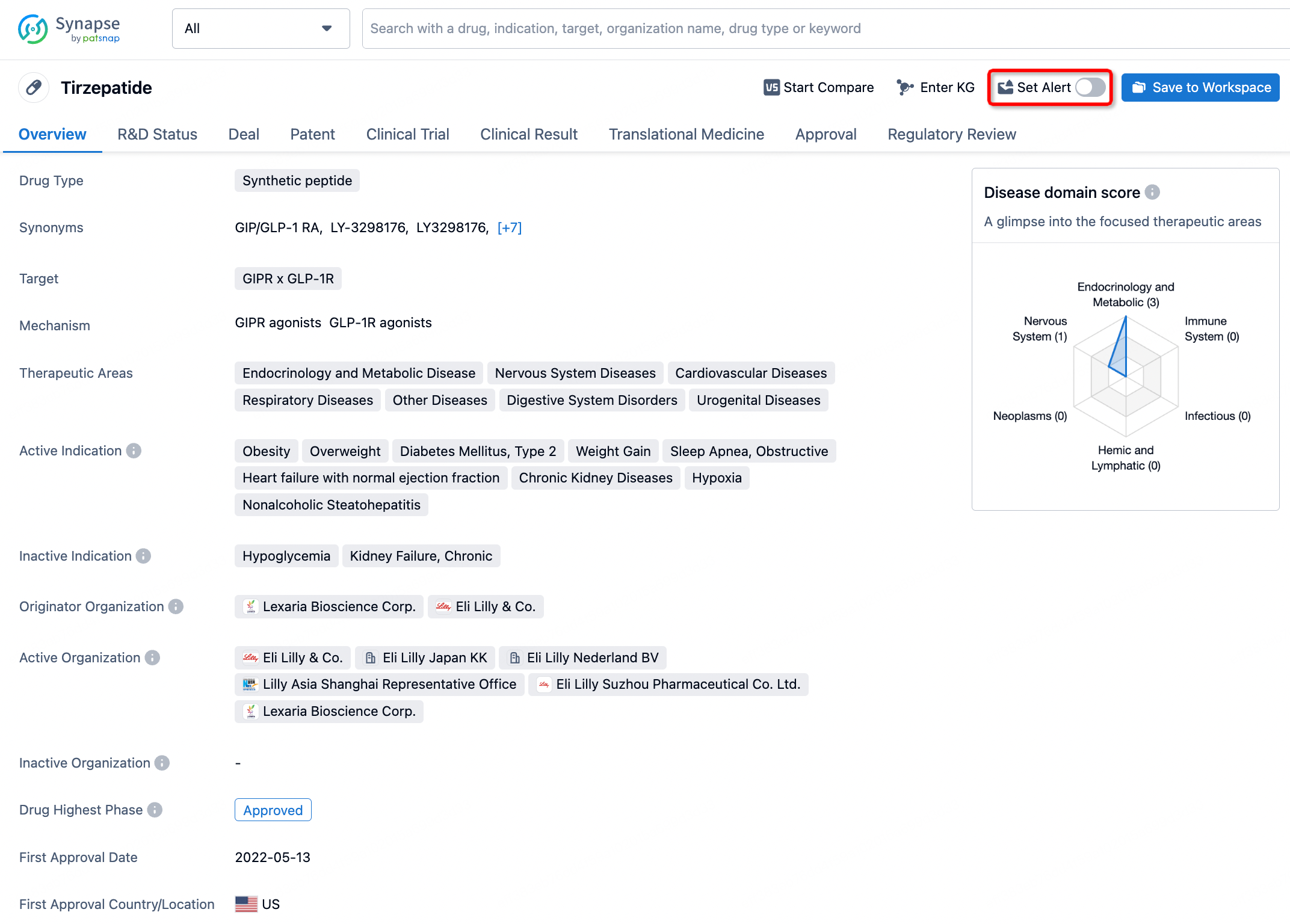
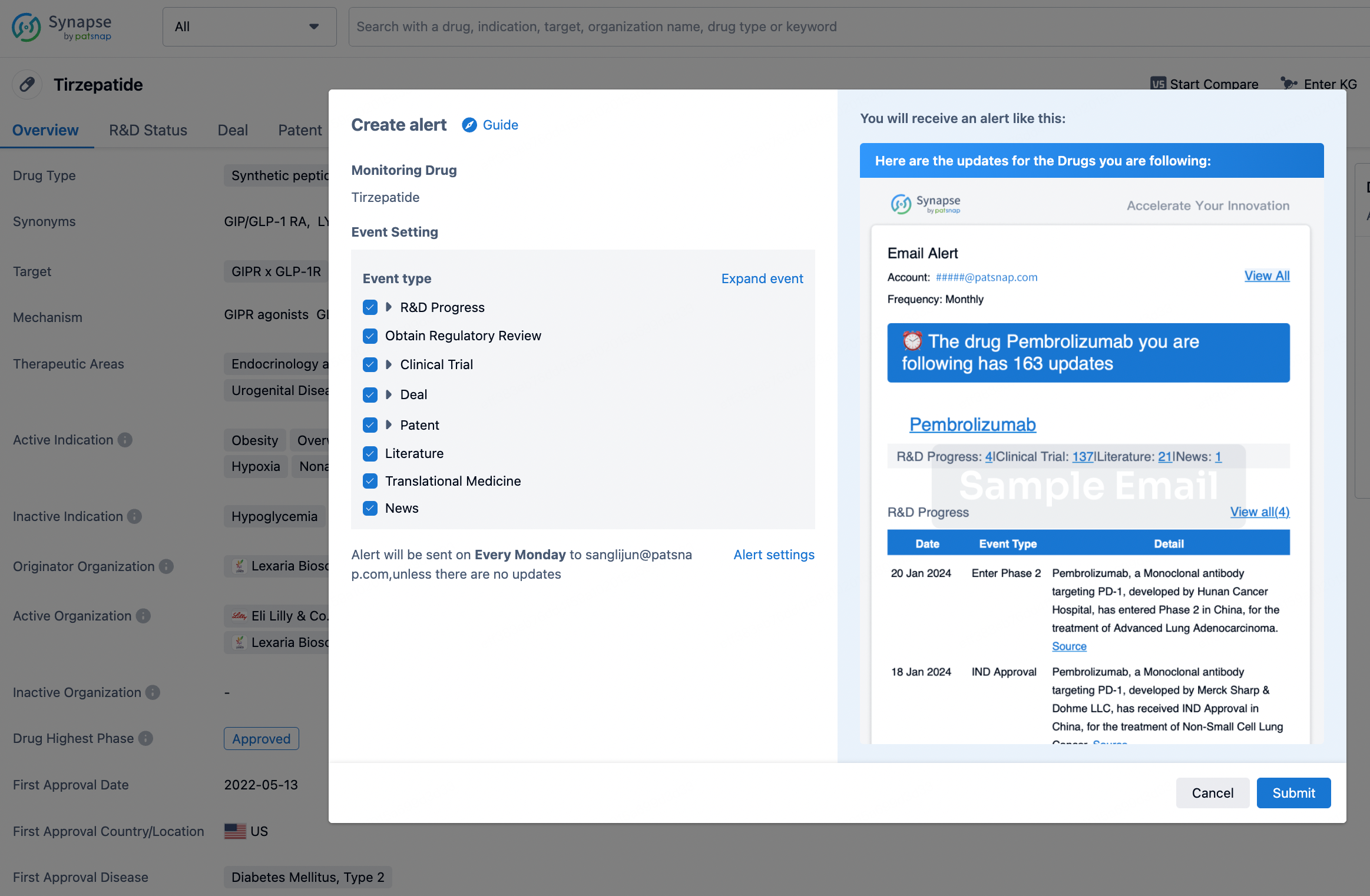
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!