Inxmed has announced two sets of data on FAK inhibitor Ifebemtinib
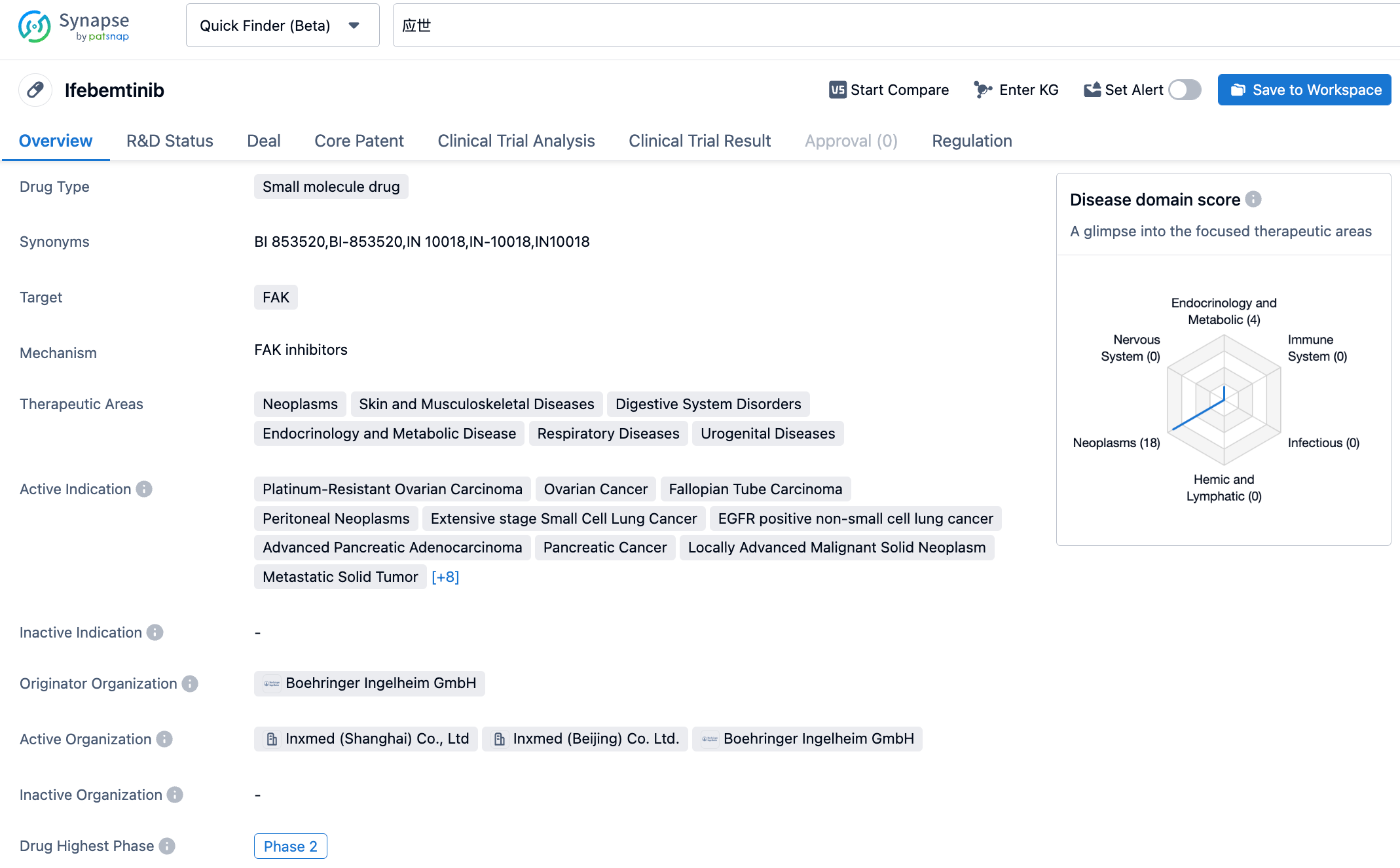
On October 23, 2023, Inxmed Co. announced that the significant clinical research results of its independently developed core product, Ifebemtinib (IN10018), the world's first small molecule FAK inhibitor, were revealed in poster form at the European Society for Medical Oncology (ESMO 2023) held in Madrid, Spain. The data showed that different combination treatment strategies centered on IN10018 demonstrated promising anti-tumor efficacy in two clinical studies on platinum-resistant recurrent ovarian cancer patients and triple-negative breast cancer patients, showing a significant trend of survival benefit.
Ifebemtinib (IN10018) is a highly efficient and selective FAK inhibitor developed by Inxmed Co. Preliminary clinical data of IN10018 demonstrated its good safety profile and efficacy in multiple types of cancers. The latest research results suggest that IN10018 could potentially overcome tumor stromal fibrosis and modulate the immunosuppressive microenvironmental aspects of the tumor, thereby effectively overcoming resistance and metastasis, and serving as a cornerstone molecule in various treatment strategies. IN10018 is under clinical development for a variety of indications, including platinum-resistant recurrent ovarian cancer, triple-negative breast cancer, non-small cell lung cancer, small cell lung cancer, pancreatic cancer and KRAS G12C mutation tumors, and the preliminary clinical findings have been accepted and presented on numerous top international and domestic oncology conferences, such as ASCO, CSCO, SMR, ESMO, and received the Fast-track Designation from the US FDA and the Breakthrough Therapy Designation from China's NMPA.
The first of the two clinical studies was a phase Ib trial on the use of IN10018 in combination with pegylated liposomal doxorubicin (PLD) for the treatment of platinum-resistant recurrent ovarian cancer. This study's primary endpoint was the Objective Response Rate (ORR), with secondary endpoints including the Disease Control Rate (DCR), the Duration of Response (DOR), Progression-Free Survival (PFS) and Overall Survival (OS). Currently, treatment methods for platinum-resistant ovarian cancer are limited, and PLD is often one of the standard chemotherapy regimens chosen. Previous studies showed that the ORR of PLD monotherapy was approximately 10%, the PFS was around 3.3 months, and the OS was around 12 months. In this study, the combination of IN10018 and PLD demonstrated very significant anti-tumor efficacy, especially in median PFS and OS, both exhibiting significant survival benefit trends.
The other trial was a phase Ib/II study on the application of IN10018 in combination with PLD and atezolizumab for the treatment of advanced triple-negative breast cancer (TNBC), aimed at evaluating the anti-tumor efficacy, safety, and tolerance of two- and three-drug combination of IN10018 in patients with locally advanced or metastatic TNBC who previously failed 1-2 standard treatments and were unable to have surgical treatment. This was the first disclosure of the clinical research results of IN10018 on this indication by the company.
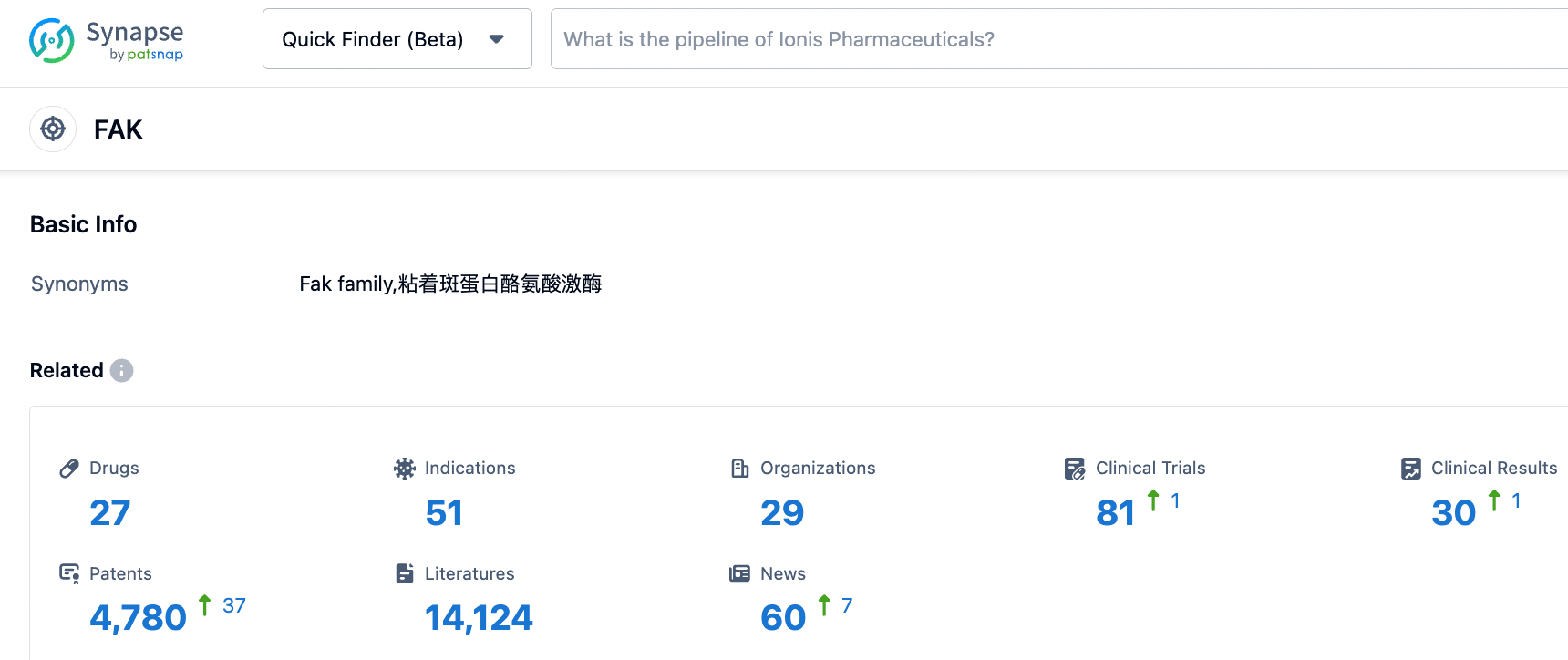
According to the information disclosed by Synapse database, as of October 24, 2023, there are 27 drugs under development for the FAK target, covering 49 indications, developed by 29 institutions, involving 81 clinical trials and up to 4779 patents....Inxmed Co. enjoys exclusive global development and commercialization rights for IN10018. Based on the impressive early-stage clinical data, IN10018 has received both Fast-track Designation from the FDA and Breakthrough Therapy Designation from NMPA. The most advanced key registration clinical trial is underway, with the New Drug Application (NDA) expected to be submitted in 2024. We look forward to a smooth research and development process for IN10018.