Novo Nordisk unveils Wegovy® cardiac outcomes at AHA meeting and in NEJM
Novo Nordisk has revealed the main findings from the SELECT trial, a significant phase 3 cardiovascular outcomes examination scrutinizing the impact of semaglutide 2.4 mg (Wegovy®), administered on a weekly basis, in adults with pre-existing cardiovascular illnesses and either overweight or obesity without concurrent diabetes. The presentation took place at the American Heart Association’s annual Scientific Sessions based in Philadelphia. Alongside the presentation, the results were also concurrently published in the New England Journal of Medicine.
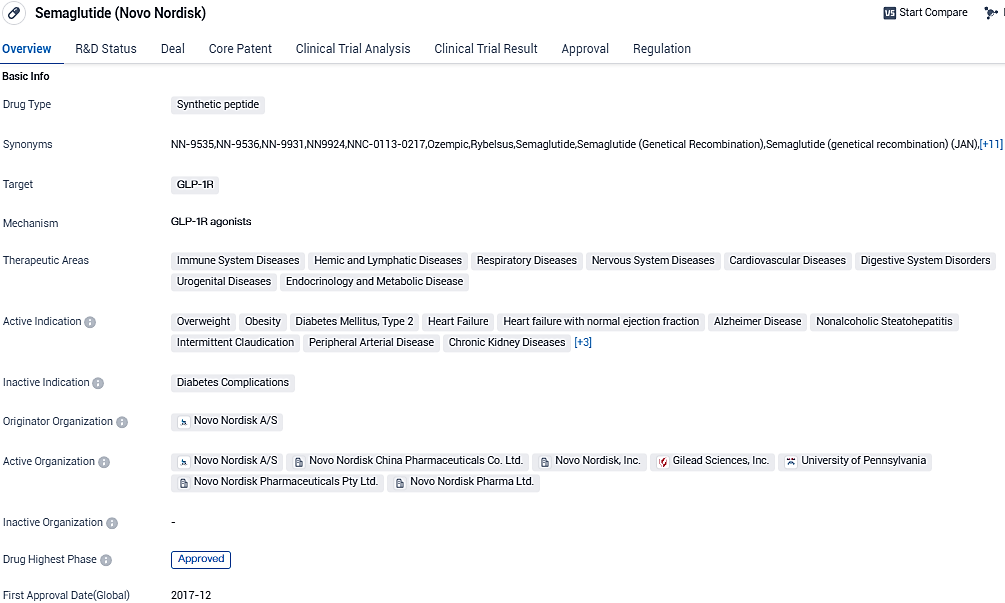
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Initial topline results indicated a significant 20% reduction in MACE risk with the use of semaglutide 2.4 mg over a five-year duration compared to a placebo. The studies revealed that the decrease in MACE risk was consistent regardless of factors such as age, gender, ethnicity, and initial body mass index (BMI).
The data also showcased that the MACE risk reduction benefits became noticeable shortly after beginning the treatment. This implies a faster effect than usual, indicating that the weight loss effects of semaglutide 2.4 mg may not be the sole explanation for the reduced MACE risk.
Cardiovascular disease (CVD) claims nearly 18 million lives each year, making it the chief cause of worldwide disability and death. Even though cardiovascular mortality has lessened over the past 20 years, deaths associated with obesity-related cardiovascular diseases have definitely surged. Obesity triggers cardiovascular illnesses and fatalities and is often linked to risks like high blood pressure and inflammation.
"This is the first instance where we've seen that semaglutide 2.4 mg enhances cardiovascular outcomes in high-risk patients with a BMI of 27 and above, who have established CVD but do not have diabetes," stated Dr Michael Lincoff, the study's principal author and vice chair for research at Cleveland Clinic's Cardiovascular Medicine Department. "The three-point MACE risk reduction seen in the SELECT study hints at a promising new obesity treatment option, addressing some of the top preventable death causes globally.”
“This significant study is the culmination of more than two decades of obesity research, a chronic condition linked with severe co-morbidities and outcomes," stated Martin Lange, the executive vice president and head of Development at Novo Nordisk. "The outcomes from the SELECT study will be a gamechanger in how we perceive and manage obesity.
These findings serve as a turning point for individuals with obesity and for the worldwide scientific community as we anticipate a new phase in obesity management and a potential decrease in cardiovascular risks with semaglutide 2.4 mg."
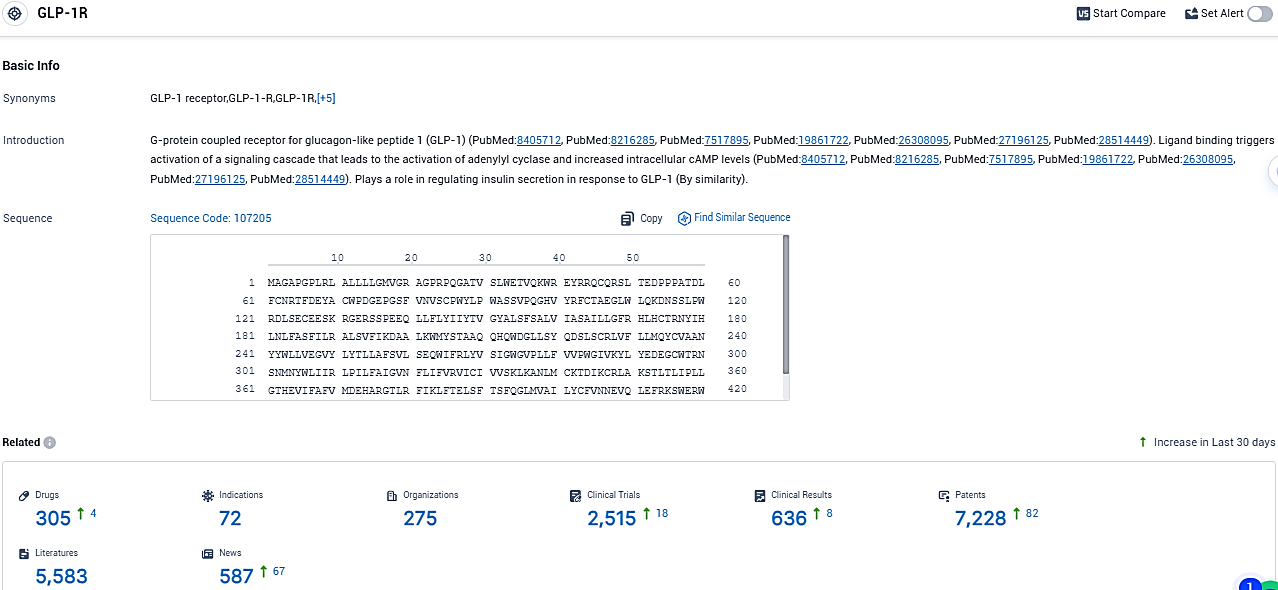
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 19, 2023, there are 305 investigational drugs for the GLP-1R target, including 72 indications, 275 R&D institutions involved, with related clinical trials reaching 2515, and as many as 636 patents.
Semaglutide targets the GLP-1R receptor. It has been approved for multiple therapeutic areas, including diabetes, obesity, heart failure, Alzheimer's disease, and pulmonary diseases. Its breakthrough therapy designation and successful global approvals highlight its potential to revolutionize the treatment landscape for various conditions.