Is Aducanumab approved by the FDA?
Aducanumab, sold under the brand name Aduhelm, is an intravenous medication used to treat Alzheimer's disease. Aducanumab was approved by the U.S. Food and Drug Administration (FDA) on June 7, 2021. This approval is notable because Aducanumab is the first treatment approved for Alzheimer's disease that targets and reduces amyloid-beta plaques in the brain, a hallmark of the disease.
Indications and Usage
Aducanumab is indicated for the treatment of Alzheimer's disease in patients with mild cognitive impairment or at the mild dementia stage of the disease. The drug works by targeting amyloid-beta plaques in the brain, which are believed to contribute to the progression of Alzheimer's.
Administration
Aducanumab is administered as an intravenous infusion:
- Frequency: Once every four weeks, with each infusion session lasting about an hour.
- Dosing: The treatment begins with a titration phase, starting with lower doses and gradually increasing to the maintenance dose of 10 mg/kg every four weeks.
- Monitoring: Patients will need regular brain MRIs to monitor for Amyloid Related Imaging Abnormalities (ARIA), a potential side effect involving brain swelling or bleeding.
Side Effects
Common side effects of Aducanumab include:
- Headache
- ARIA, which may present with symptoms or be detected on an MRI
- Falls
Severe side effects may include:
- Allergic reactions (hives, difficulty breathing, swelling)
- Brain swelling or bleeding (dizziness, confusion, trouble walking, seizures, nausea, vision changes)
Patients should seek immediate medical attention if they experience severe side effects.
Warnings and Precautions
ARIA Risk: Aducanumab can cause ARIA, which typically resolves over time but can be serious. Symptoms include headache, dizziness, nausea, confusion, and vision changes.
Pregnancy and Breastfeeding: It is not known if Aducanumab affects an unborn baby or passes into breast milk. Patients should inform their healthcare provider if they are pregnant or planning to become pregnant.
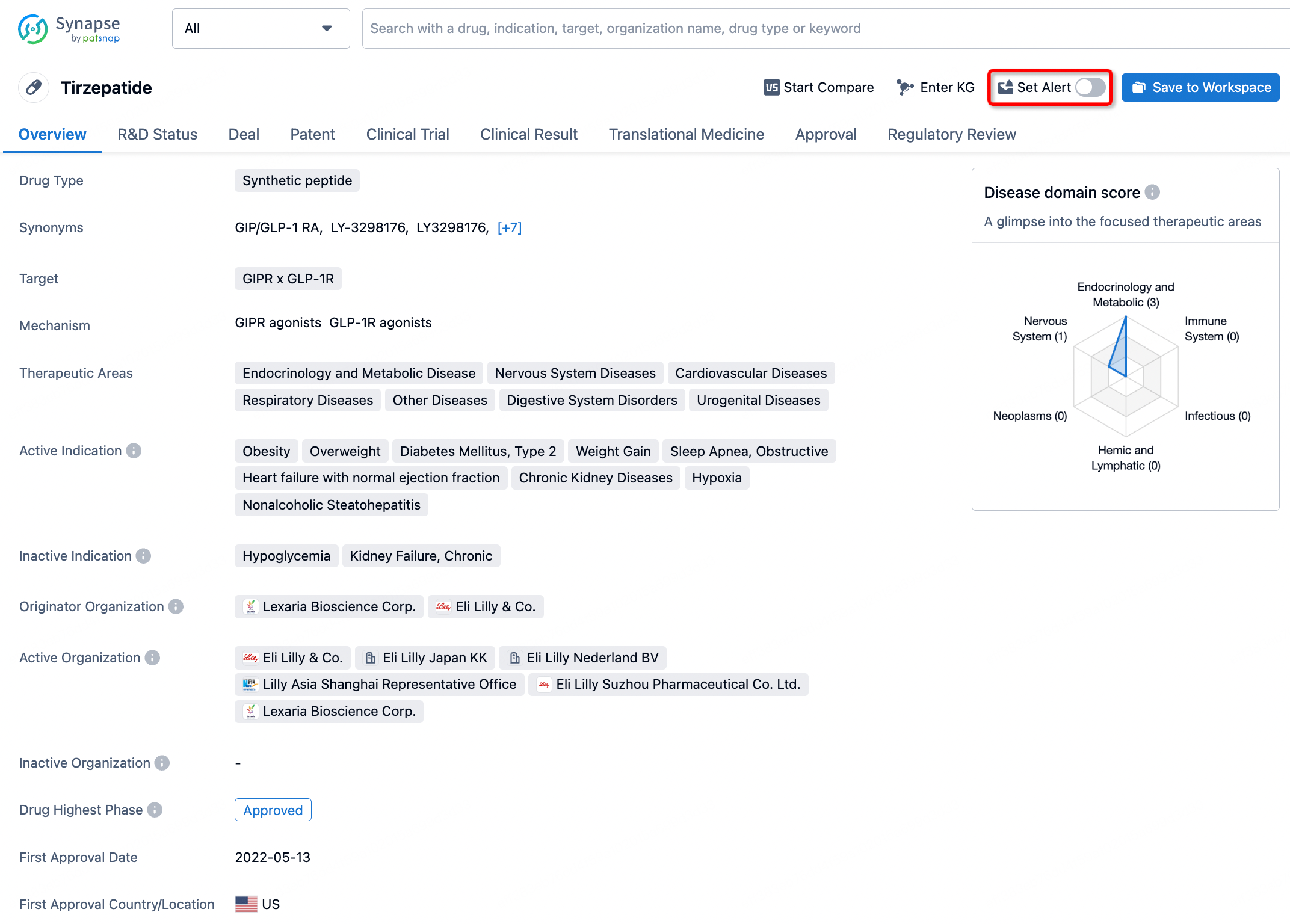
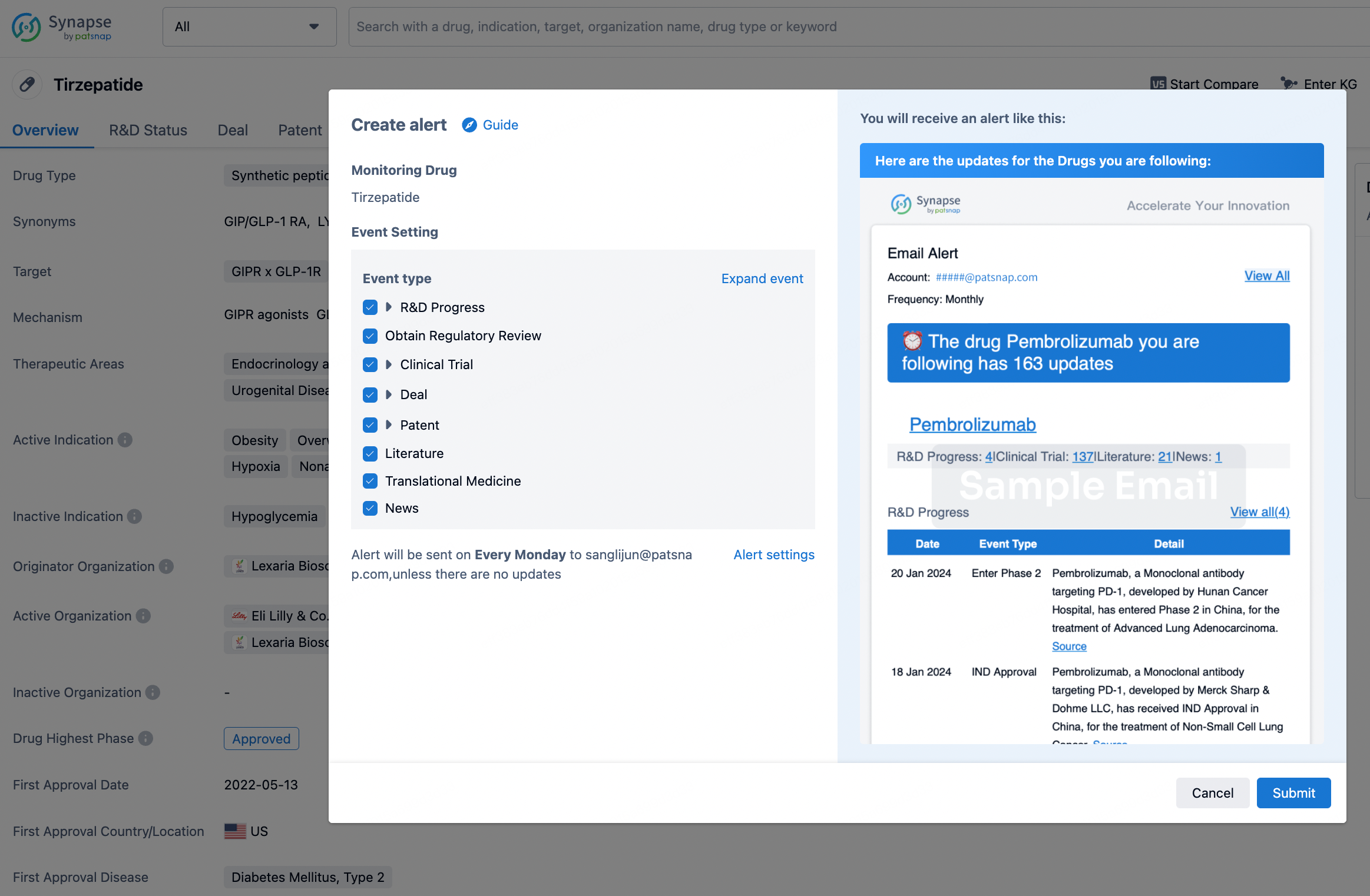
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!