Is Finerenone approved by the FDA?
Yes, finerenone (Kerendia) is FDA approved. The U.S. Food and Drug Administration (FDA) approved finerenone on July 9, 2021. This approval is based on its efficacy in reducing the risk of kidney function decline, cardiovascular events, and heart failure hospitalizations in patients with CKD associated with type 2 diabetes.
Uses and Benefits
Finerenone is used to reduce the risk of:
- Worsening kidney problems
- Heart attacks
- Hospitalization for heart failure
- Death from heart failure
Administration and Dosage
Finerenone is available in oral tablet form, typically prescribed in doses of 10 mg or 20 mg. It can be taken with or without food. For patients with an estimated glomerular filtration rate (eGFR) of at least 60 mL/min/1.73 m², the usual starting dose is 20 mg once a day. For those with an eGFR of 25 to less than 60 mL/min/1.73 m², the starting dose is 10 mg once a day, with a target dose of 20 mg daily.
Side Effects
Common side effects of finerenone include:
- High potassium levels (hyperkalemia)
- Low sodium levels (hyponatremia)
- Low blood pressure (hypotension)
Serious side effects requiring immediate medical attention include:
- Allergic reactions (hives, difficulty breathing, swelling of face, lips, tongue, or throat)
- Symptoms of high blood potassium (nausea, weakness, tingling, chest pain, irregular heartbeats)
- Symptoms of low blood sodium (headache, confusion, memory problems, weakness, unsteady feeling)
Warnings and Precautions
Finerenone should not be used by individuals who are allergic to it or have adrenal gland issues. Patients should inform their doctor about all other medications they are taking, as certain drugs should not be used with finerenone due to potential interactions. Additionally, it is important to avoid grapefruit products while taking finerenone, as grapefruit can interact with the medication and cause side effects.
Conclusion
Finerenone is an FDA-approved medication effective in managing chronic kidney disease associated with type 2 diabetes, reducing risks of kidney and cardiovascular complications. It is essential to use this medication under medical supervision due to its potential side effects and interactions with other drugs.
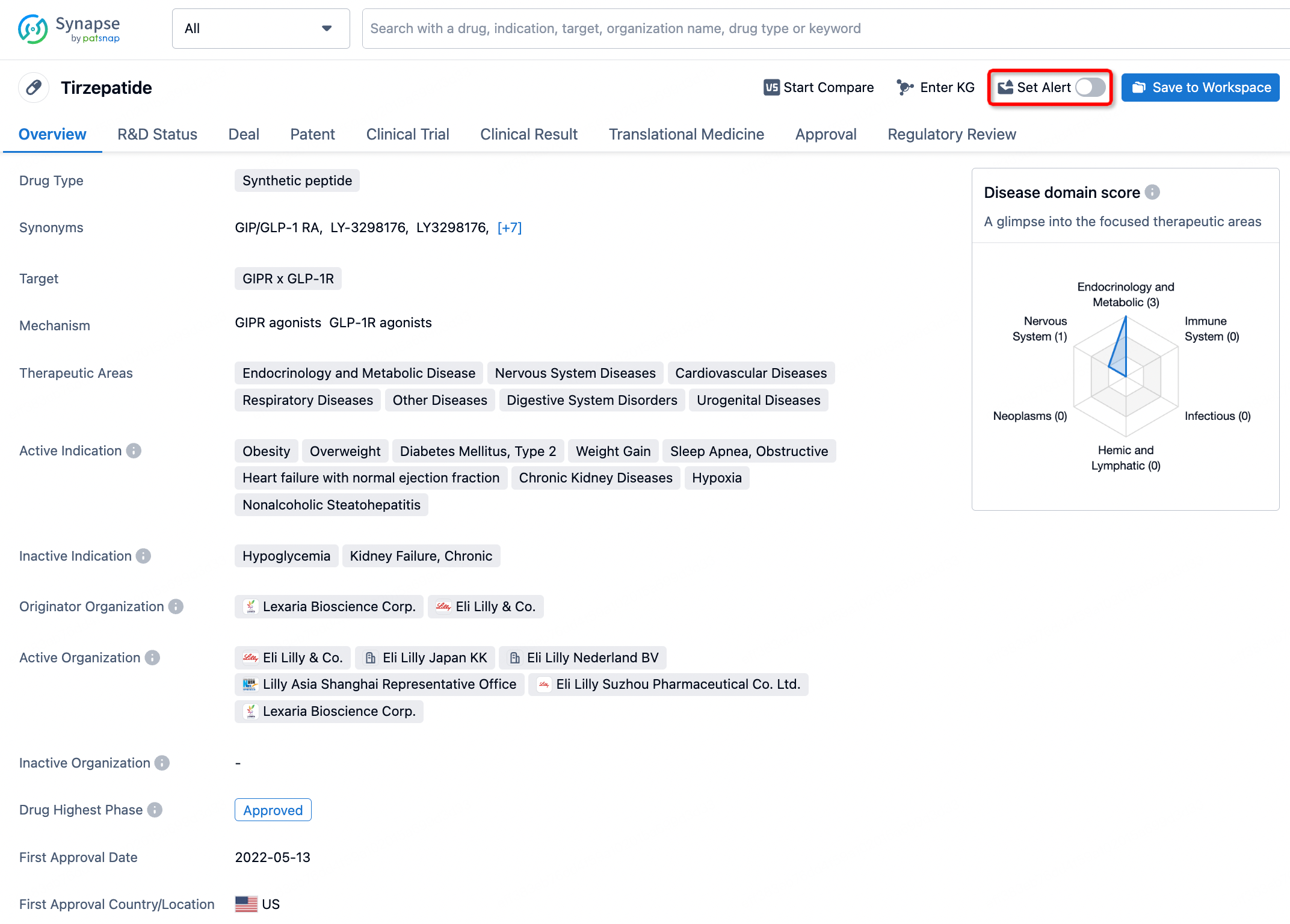
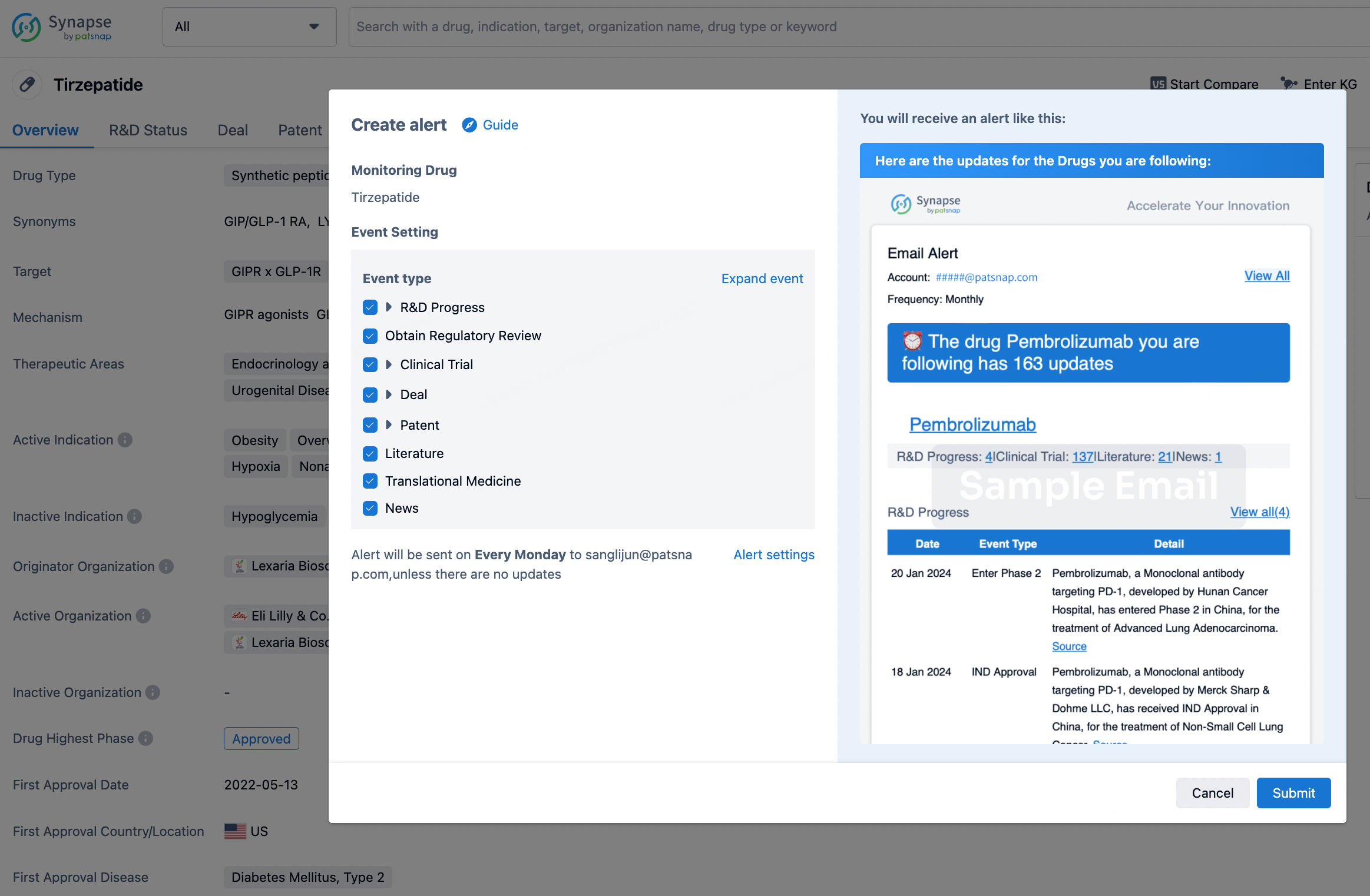
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!