Is Futibatinib approved by the FDA?
Futibatinib (brand name: Lytgobi) is an FDA-approved medication used to treat certain types of bile duct cancer (cholangiocarcinoma). Futibatinib was approved by the FDA on September 30, 2022.Specifically, it is indicated for adults with bile duct cancer that has spread (metastatic) or cannot be surgically removed, who have already undergone another treatment, and whose tumor has an abnormal "FGFR2" gene.
What is Futibatinib?
Futibatinib is an oral multikinase inhibitor that targets and inhibits the activity of FGFR2, a protein involved in the growth and spread of cancer cells. By blocking this protein, futibatinib helps to slow or stop the progression of bile duct cancer.
Dosage and Administration:
Futibatinib is available in oral tablet form with the following dosages:
- Daily Dose: 20 mg (five 4 mg tablets) taken orally once daily.
Patients should swallow the tablet whole without crushing, chewing, or breaking it. The medication can be taken with or without food, ideally at the same time each day. If a dose is missed by more than 12 hours, patients should skip the missed dose and continue with the next scheduled dose.
Important Considerations:
- Confirm the presence of an FGFR2 gene fusion or rearrangement before starting treatment.
- Continue treatment until disease progression or unacceptable toxicity occurs.
- Regular eye exams are necessary before starting treatment, every 2 months for the first 6 months, and every 3 months thereafter.
- Blood tests are required during treatment to monitor for side effects.
Common Side Effects:
Futibatinib may cause various side effects, some of which can be serious. Common side effects include:
- Stomach pain, nausea, vomiting, constipation, diarrhea, loss of appetite
- Dry mouth, skin, or eyes
- Pain and burning during urination
- Tiredness or weakness
- Redness, swelling, pain, or blisters on the palms of the hands or soles of the feet
- Muscle or joint pain
- Changes in taste, appearance, or color of nails
- Hair loss
- Abnormal lab tests
- High blood sugar
- Low blood sugar
- Low blood potassium
- High calcium levels
- Low blood sodium
- Low blood cell counts
Serious Side Effects:
Patients should seek immediate medical attention if they experience serious side effects such as:
- Vision changes, including blurred vision, seeing flashes of light, or black spots
- Muscle cramps
- Numbness or tingling around the mouth
- Kidney problems
- Seizures
- Light-headed feeling, as if about to pass out
Warnings:
- Futibatinib can harm an unborn baby. Women should use birth control during treatment and for at least 1 week after the last dose. Men should use birth control if their partner can become pregnant, continuing for at least 1 week after the last dose.
- Do not breastfeed while using futibatinib and for at least 1 week after the last dose.
- Avoid grapefruit products as they may interact with the medication.
Conclusion:
Futibatinib (Lytgobi) is an FDA-approved treatment for bile duct cancer with specific genetic mutations. Approved on September 30, 2022, this medication offers a targeted approach for managing advanced or metastatic bile duct cancer, providing hope for patients with limited treatment options. Patients should closely follow their healthcare provider's instructions and undergo regular monitoring to manage potential side effects and ensure the effectiveness of the treatment.
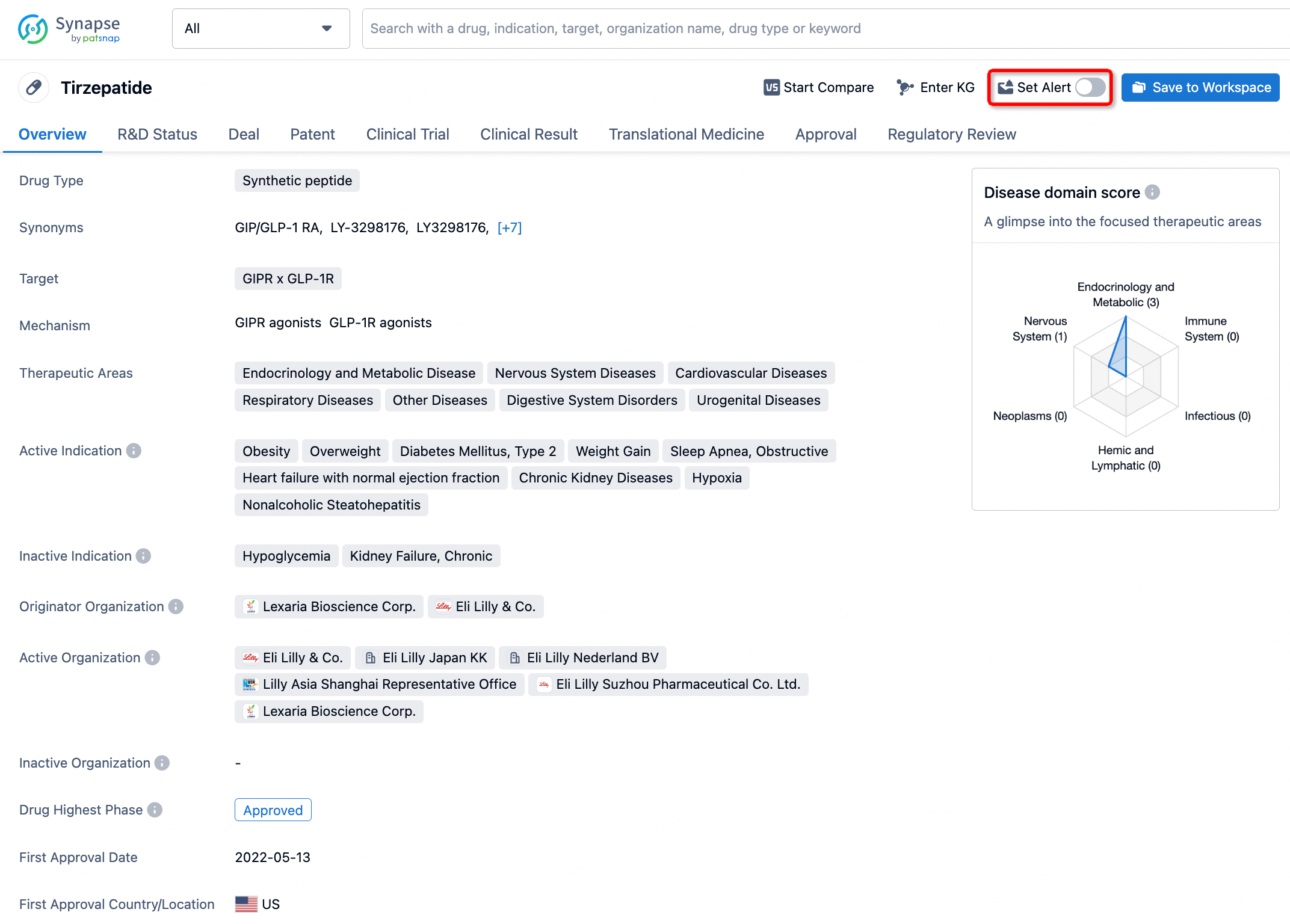
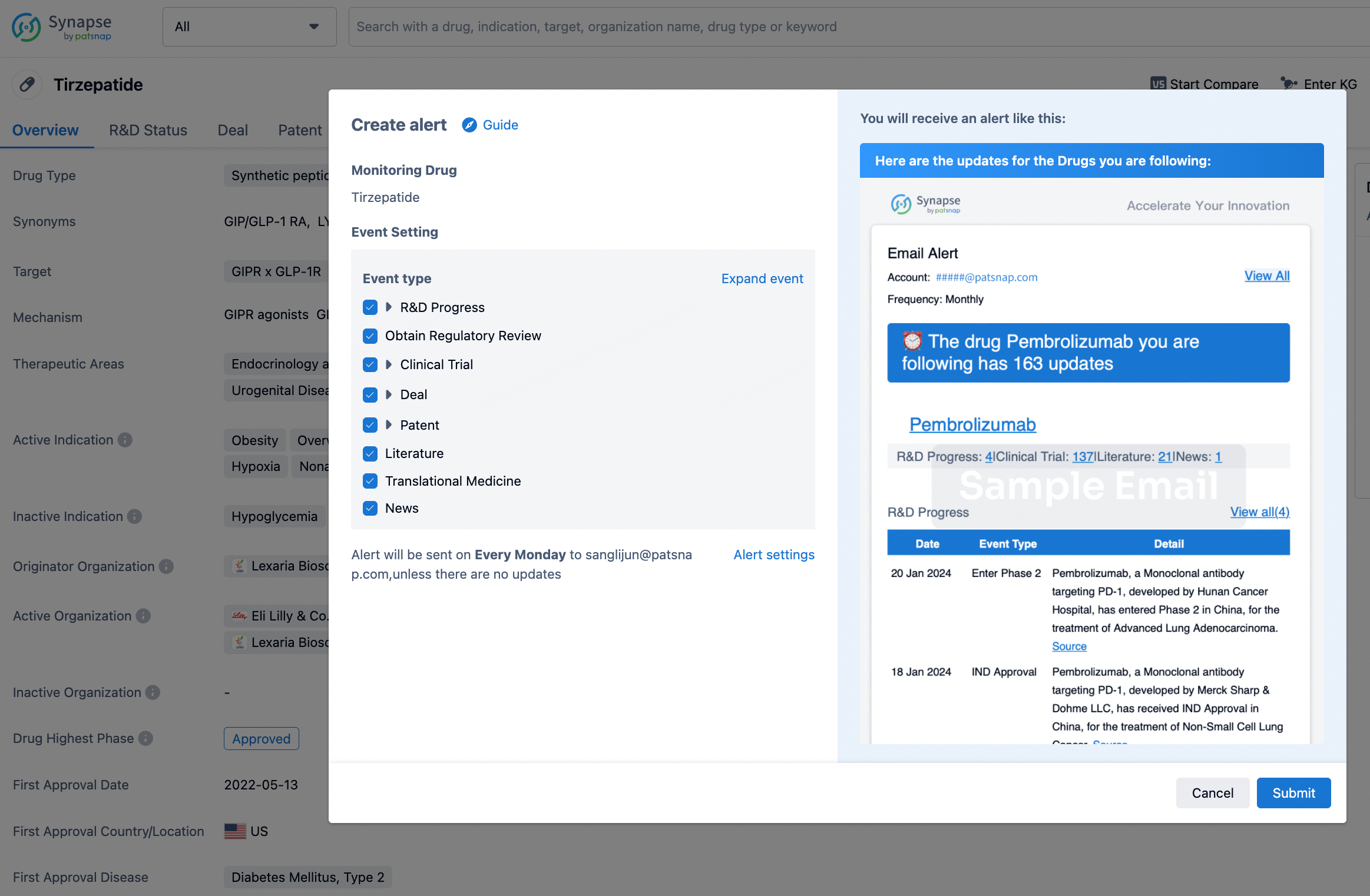
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!