Is Rozanolixizumab approved by the FDA?
Rozanolixizumab, marketed under the brand name Rystiggo, was approved by the FDA on June 26, 2023, for the treatment of generalized myasthenia gravis in adult patients who are anti-acetylcholine receptor (AChR) or anti-muscle-specific tyrosine kinase (MuSK) antibody positive. This approval provides a new therapeutic option for patients suffering from this debilitating condition.
Uses and Administration
- Indication: Rozanolixizumab is used to treat generalized myasthenia gravis in adults.
- Administration: The medication is administered as a subcutaneous infusion once a week for six weeks. The dosage is based on body weight, with the following guidelines:
- Less than 50 kg: 420 mg (3 mL) once weekly
- 50 kg to less than 100 kg: 560 mg (4 mL) once weekly
- 100 kg and above: 840 mg (6 mL) once weekly The infusion should be set at a rate of up to 20 mL per hour and administered using an infusion pump. Additional treatment cycles may be considered based on clinical assessment, but the safety of initiating subsequent cycles within 63 days from the beginning of the previous treatment cycle has not been confirmed.
Side Effects
While rozanolixizumab can be effective in treating myasthenia gravis, it may cause various side effects. Patients should be aware of both common and serious side effects and seek medical attention if necessary.
Common Side Effects:
- Headache
- Diarrhea
- Fever
- Nausea
- Pain, bruising, swelling, or irritation at the injection site
- Any type of infection
Serious Side Effects:
- Signs of infection: fever, chills, sore throat, mouth sores, red or swollen gums, pale skin, easy bruising, unusual bleeding, chest discomfort, wheezing, dry cough, rapid weight loss
- Signs of meningitis: headache, neck stiffness, increased sensitivity to light, nausea, vomiting, confusion, drowsiness
Patients experiencing any severe side effects should seek emergency medical help immediately.
Precautions and Warnings
- Infections: Rozanolixizumab can increase the risk of serious or fatal infections. Patients should inform their doctor about any signs of infection or a history of infections. Blood tests for infections are required before starting treatment.
- Allergies: Patients allergic to rozanolixizumab should not use this medication.
- Vaccinations: Patients should be up to date on all vaccines before starting rozanolixizumab and avoid live vaccines during treatment.
- Pregnancy and Breastfeeding: It is not known if rozanolixizumab will harm an unborn baby. Patients should inform their doctor if they are pregnant or plan to become pregnant. Breastfeeding should be avoided while using rozanolixizumab and for at least 14 hours after the last dose.
Drug Interactions
Rozanolixizumab can interact with other medications, including prescription drugs, over-the-counter medicines, vitamins, and herbal products. Patients should inform their doctor about all other medicines they are using to avoid potential interactions.
Storage
Store rozanolixizumab at room temperature, away from moisture and heat. Keep the medication in its original container until it is time to use it.
Conclusion
Rozanolixizumab (Rystiggo) is an FDA-approved treatment for generalized myasthenia gravis in adults, providing a new therapeutic option for managing this condition. Approved on June 26, 2023, rozanolixizumab offers hope for patients with this chronic autoimmune disorder. As with any medication, it is important to be aware of potential side effects and necessary precautions. Always follow the prescribed instructions and consult with healthcare providers to ensure safe and effective use.
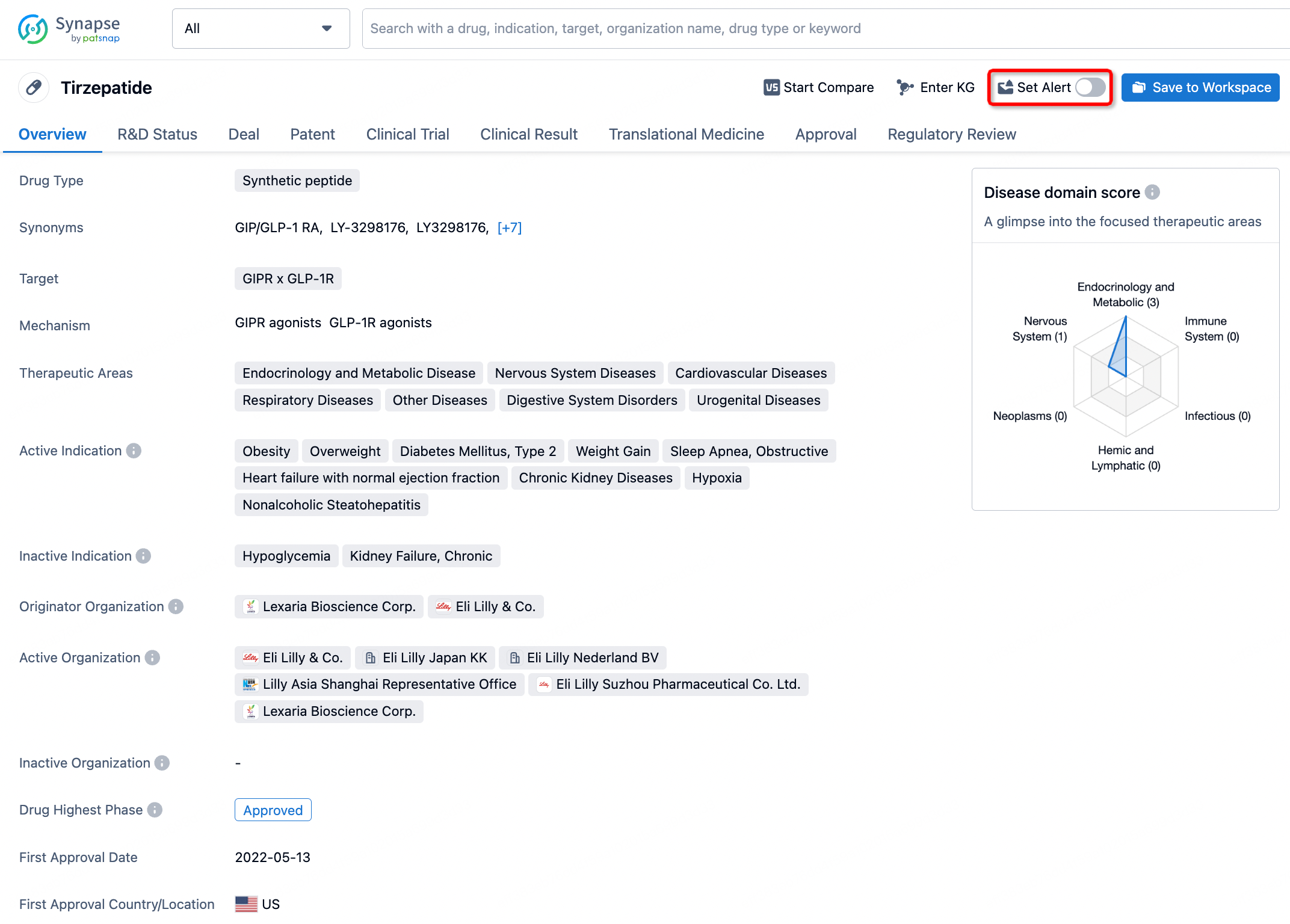
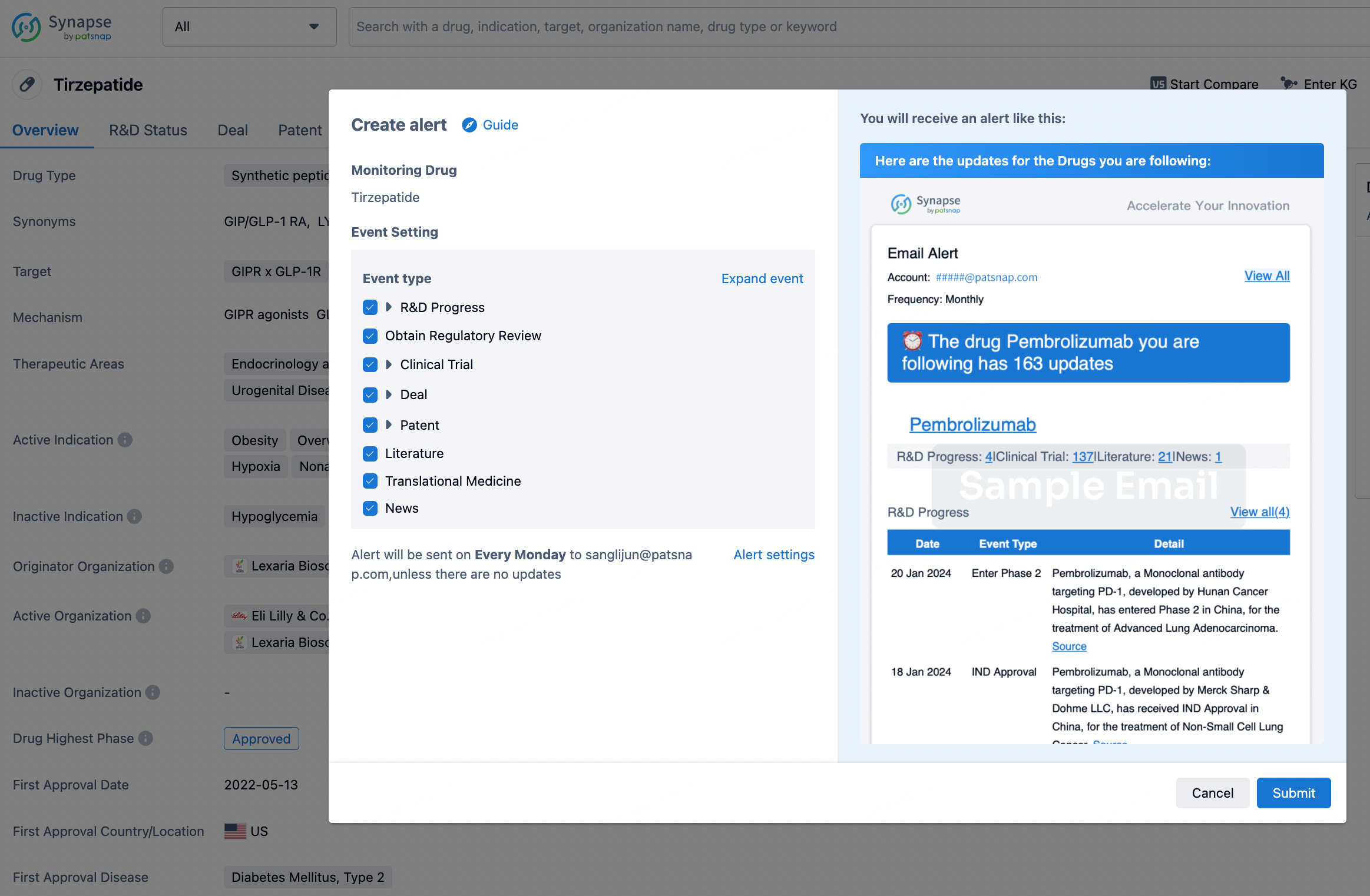
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!