Is Spesolimab approved by the FDA?
Spesolimab, marketed under the brand name Spevigo, received FDA approval for the treatment of generalized pustular psoriasis flares in adults. It belongs to the drug class of interleukin inhibitors.It is available as an intravenous solution with a concentration of sbzo 450 mg/7.5 mL.
What is Spesolimab?
Spesolimab is specifically indicated for adults suffering from generalized pustular psoriasis (GPP). This condition is characterized by widespread patches of pustules on the skin, which can be debilitating and challenging to manage.
How Spesolimab Works
Spesolimab works by targeting interleukin, a protein involved in the inflammatory response that plays a role in the development of psoriasis symptoms. By inhibiting interleukin, spesolimab helps to reduce the severity and frequency of GPP flares.
Administration and Dosage
- Dosing: The typical dose of spesolimab for GPP is 900 mg administered intravenously over a period of 90 minutes.
- Additional Dose: If symptoms persist after the initial dose, another 900 mg dose may be given one week later.
Side Effects
Common side effects of spesolimab may include:
- Fatigue or weakness
- Nausea and vomiting
- Headache
- Itching or skin reactions at the injection site
- Pain or burning during urination
Precautions and Warnings
Before starting spesolimab treatment, patients should inform their healthcare provider about any allergies, ongoing infections, or recent vaccinations. It's crucial to undergo testing for tuberculosis and other infections due to the immunosuppressive nature of spesolimab.
Conclusion
Spesolimab (Spevigo) is an FDA-approved medication designed to alleviate the symptoms of generalized pustular psoriasis in adults. It offers relief by targeting interleukin, thereby addressing the underlying inflammatory processes associated with GPP. As with any medication, it's essential for patients to follow their healthcare provider's instructions closely and report any adverse reactions promptly.
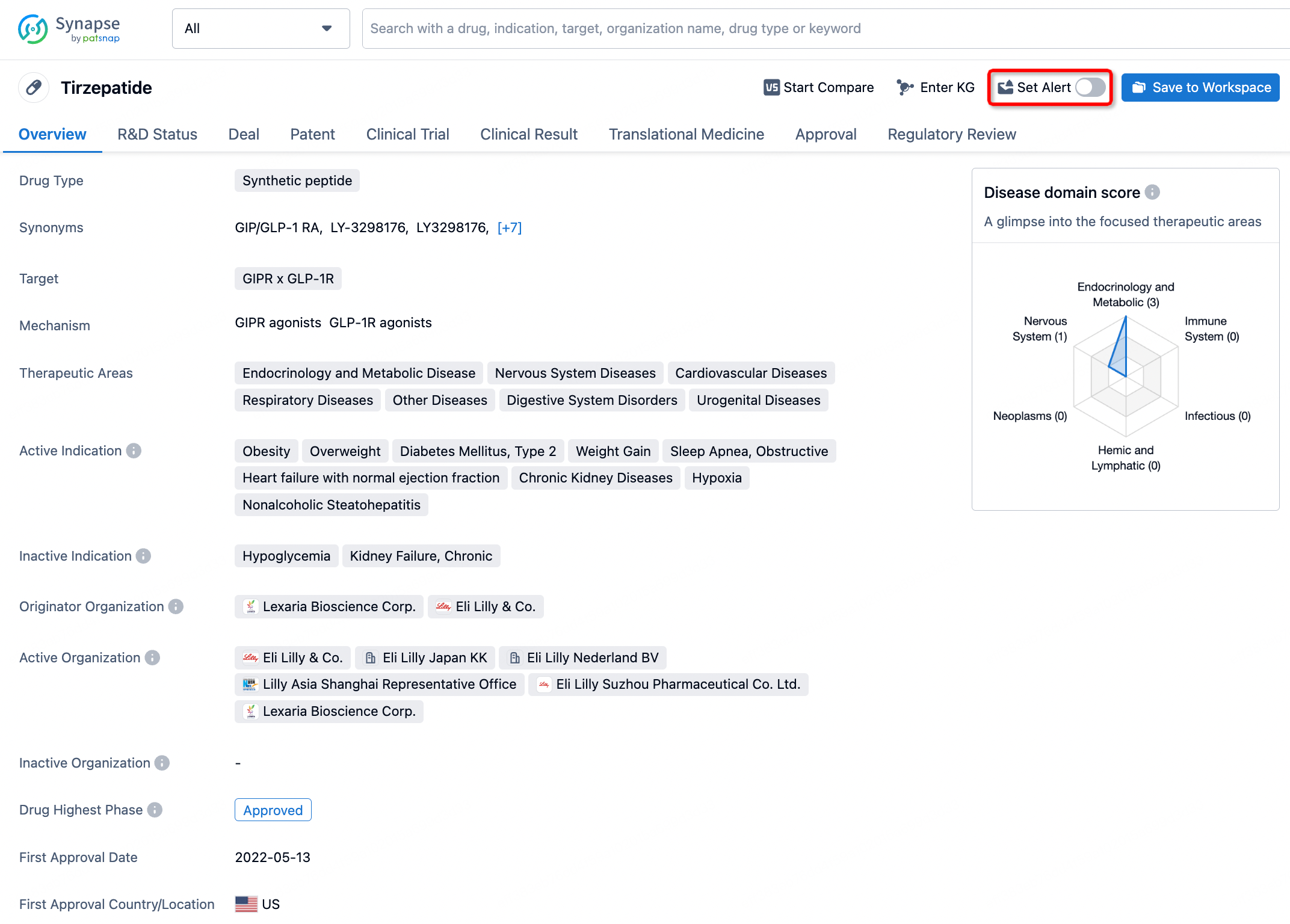
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!