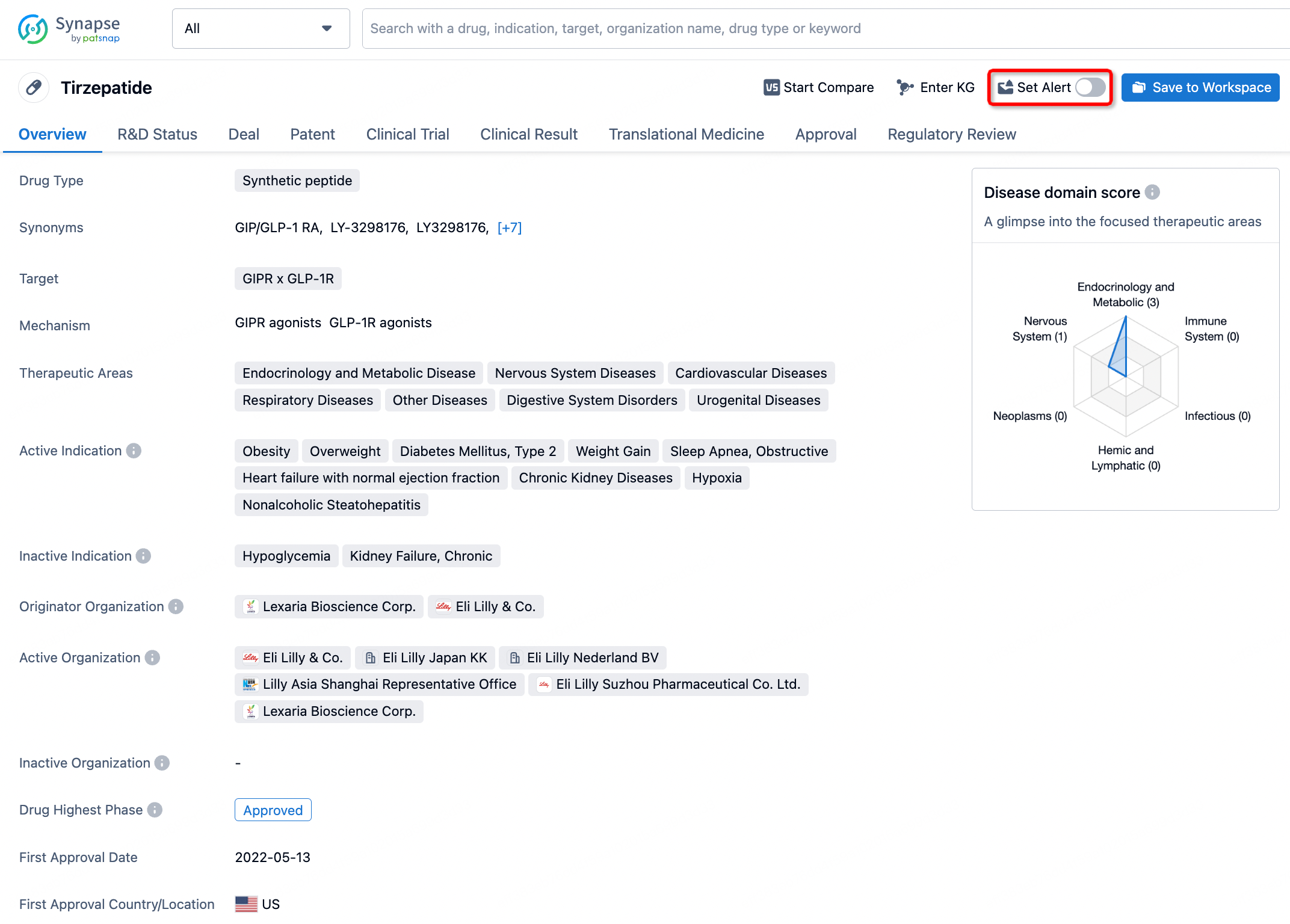
Is Tirbanibulin approved by the FDA?
Tirbanibulin, marketed under the brand name Klisyri, is a topical ointment used primarily to treat actinic keratosis, a skin condition caused by repeated exposure to ultraviolet light from the sun or indoor tanning. Tirbanibulin was approved by the US Food and Drug Administration (FDA) on December 14, 2020, for the treatment of actinic keratosis on the face or scalp.
Uses and Administration
Uses:
- Tirbanibulin is indicated for the treatment of actinic keratosis on the face or scalp.
- It is not suitable for other skin conditions or purposes not listed in the medication guide.
Administration:
- The ointment should be applied once daily for five consecutive days.
- Apply only enough medication to cover the affected area.
- Wash hands with soap and water after application.
- Avoid covering the treated skin area with a bandage.
- Do not wash or touch the treated area for at least 8 hours after application.
- Each single-dose packet is intended for one use only; discard the packet after use.
Side Effects
Common Side Effects:
- Itching or pain at the application site
Serious Side Effects:
- Redness, swelling, flaking, scaling, peeling, or crusting of treated skin
- Blisters, pus, ulcers, or breakdown of the skin
If any severe side effects occur, contact your doctor immediately. You can report side effects to the FDA at 1-800-FDA-1088.
Warnings and Precautions
- Follow all directions on your prescription label and medication guides.
- Inform your healthcare providers about all your medical conditions, allergies, and treatments.
- Delay starting tirbanibulin if you have other skin treatments until your skin has healed.
- Not approved for use by individuals under 18 years old.
- If you are pregnant or breastfeeding, consult your doctor before using tirbanibulin.
Drug Interactions
Topical medications like tirbanibulin are unlikely to interact with other drugs you use. However, it is essential to inform your healthcare providers about all the medications you are taking, including prescription drugs, over-the-counter medicines, vitamins, and herbal products.
Conclusion
This medication offers a targeted treatment option for patients with this condition. As with all medications, it is crucial to follow the prescribed instructions and consult your healthcare provider with any concerns or questions regarding its use.
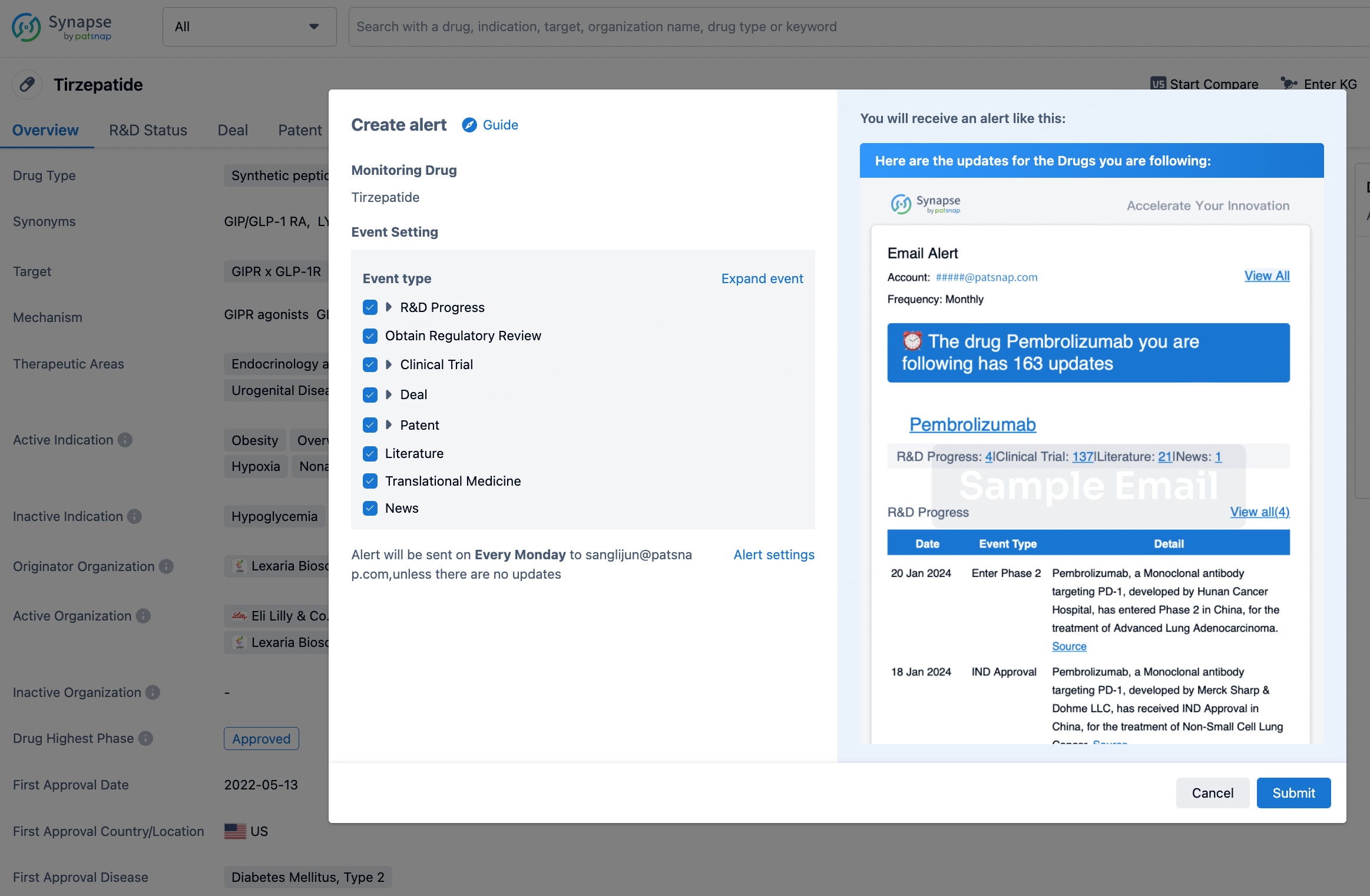
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!