Is Tucatinib approved by the FDA?
Yes, Tucatinib, marketed under the brand name Tukysa, is FDA approved. The U.S. Food and Drug Administration (FDA) granted approval for Tucatinib on April 17, 2020. Tucatinib is a HER2 inhibitor used in combination with trastuzumab and capecitabine for the treatment of HER2-positive breast cancer.
What is Tucatinib?
Tucatinib is a targeted anticancer drug that belongs to the class of tyrosine kinase inhibitors. It specifically targets the HER2 (human epidermal growth factor receptor 2) tyrosine kinase. In HER2-positive breast cancers, the HER2 gene is often faulty, leading to overexpression of HER2 proteins, which causes the cancer cells to grow and divide uncontrollably. Tucatinib works by blocking the activity of HER2, slowing the growth and spread of cancer cells.
Indications and Usage
Tucatinib is prescribed in combination with trastuzumab and capecitabine for adults with:
- HER2-positive breast cancer that has spread to other parts of the body, such as the brain (metastatic).
- HER2-positive breast cancer that cannot be removed by surgery.
- Patients who have received one or more anti-HER2 treatments for metastatic breast cancer.
Administration and Dosage
Tucatinib is taken orally as a tablet, twice daily with or without food, approximately 12 hours apart. The recommended dosage is 300 mg taken orally twice daily. For patients with severe hepatic impairment, the dosage is adjusted to 200 mg twice daily. Tucatinib should be swallowed whole and should not be chewed, crushed, or split.
Side Effects
Common side effects of Tucatinib include:
- Diarrhea
- Rash, redness, pain, swelling, or blisters on the palms or soles
- Nausea
- Fatigue
- Elevated liver function tests
- Vomiting
- Mouth sores (stomatitis)
- Decreased appetite
- Abdominal pain
- Headache
- Anemia
Serious side effects include severe diarrhea and liver problems. Patients are advised to monitor for signs of liver issues, such as itching, yellowing of the skin or eyes, dark urine, abdominal pain, fatigue, decreased appetite, and easy bruising or bleeding.
Precautions and Warnings
Patients should inform their healthcare provider about any existing medical conditions, particularly liver problems, before starting treatment with Tucatinib. Effective birth control should be used during treatment and for at least one week after the last dose due to the risk of harm to an unborn baby. Breastfeeding is not recommended during treatment and for at least one week after the last dose.
Conclusion
Tucatinib (Tukysa) is an FDA-approved medication for treating HER2-positive breast cancer, specifically in cases where the cancer is metastatic or inoperable. Approved on April 17, 2020, Tucatinib offers a targeted approach to slowing cancer progression when used in combination with trastuzumab and capecitabine. Patients should follow their healthcare provider's instructions carefully and be aware of potential side effects and necessary precautions during treatment.
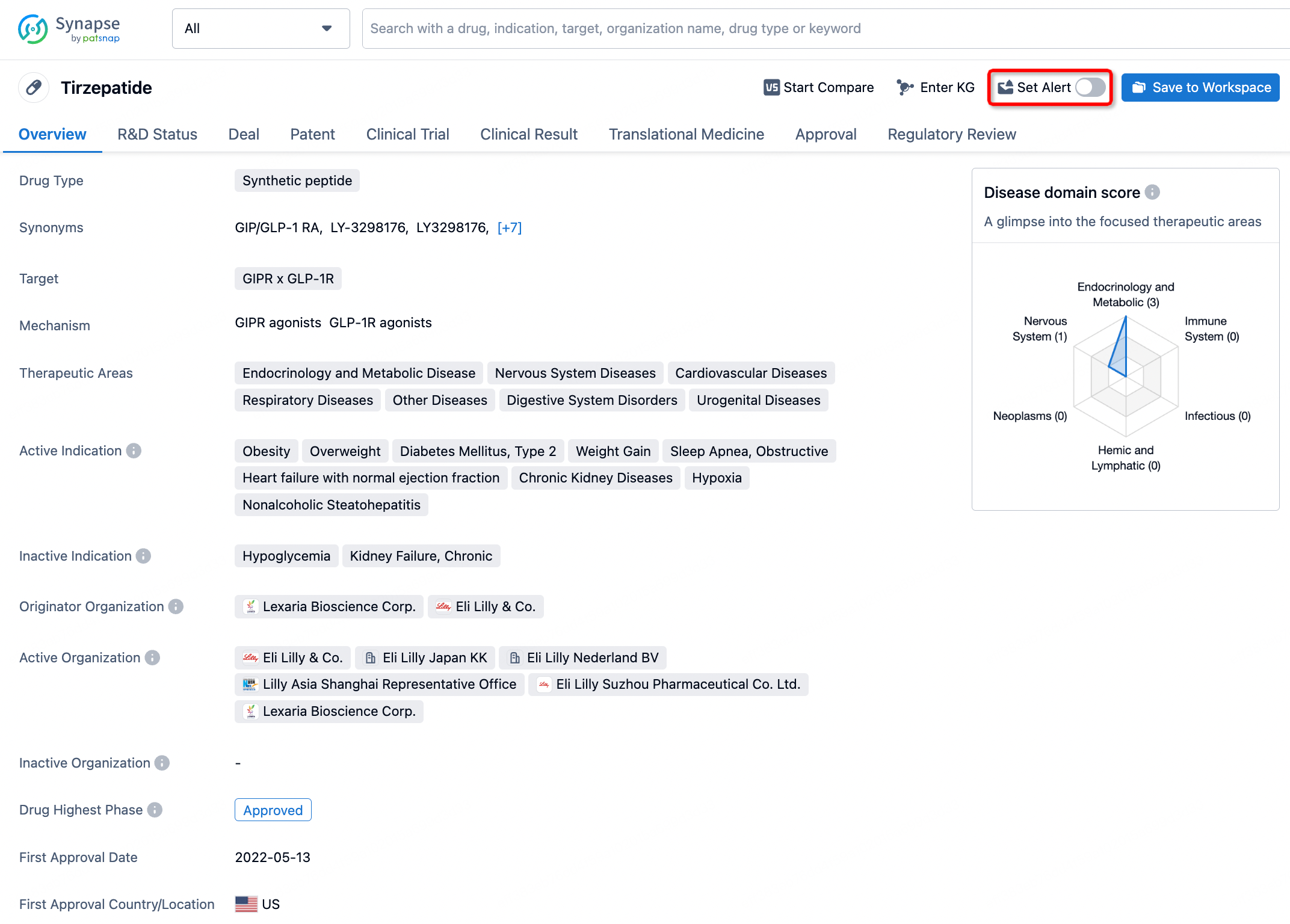
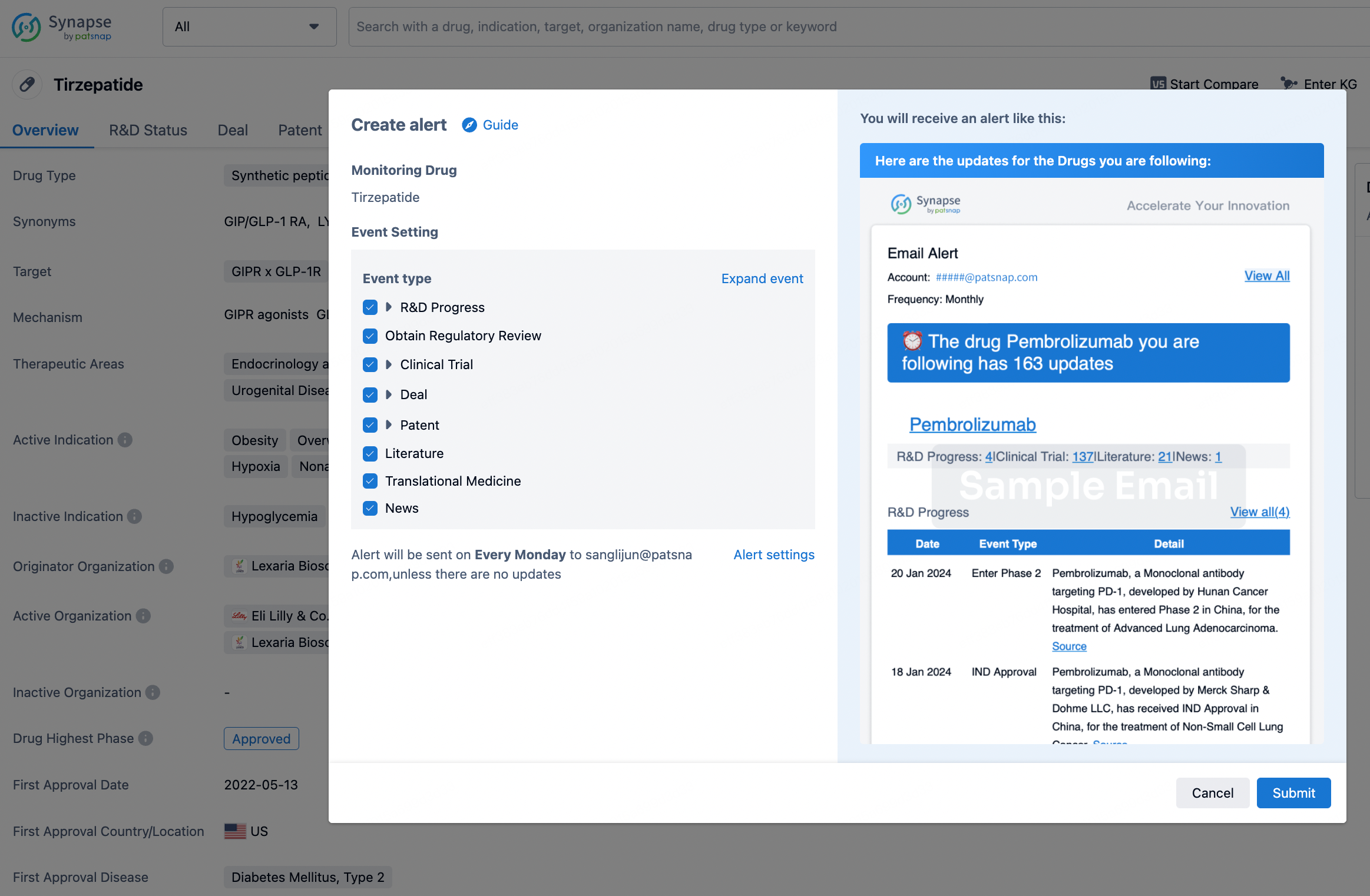
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!