J&J Seeks EMA Approval for Subcutaneous RYBREVANT® for EGFR-Mutated Lung Cancer
Johnson & Johnson's Janssen Pharmaceutical Companies have filed a type II extension of indication application with the European Medicines Agency. This application seeks approval for combining RYBREVANT® (amivantamab) with chemotherapy for treating adults with advanced non-small cell lung cancer who have either epidermal growth factor receptor (EGFR) exon 19 deletions or L858R substitution mutations, following the failure of prior treatments, including third-generation EGFR tyrosine kinase inhibitors.
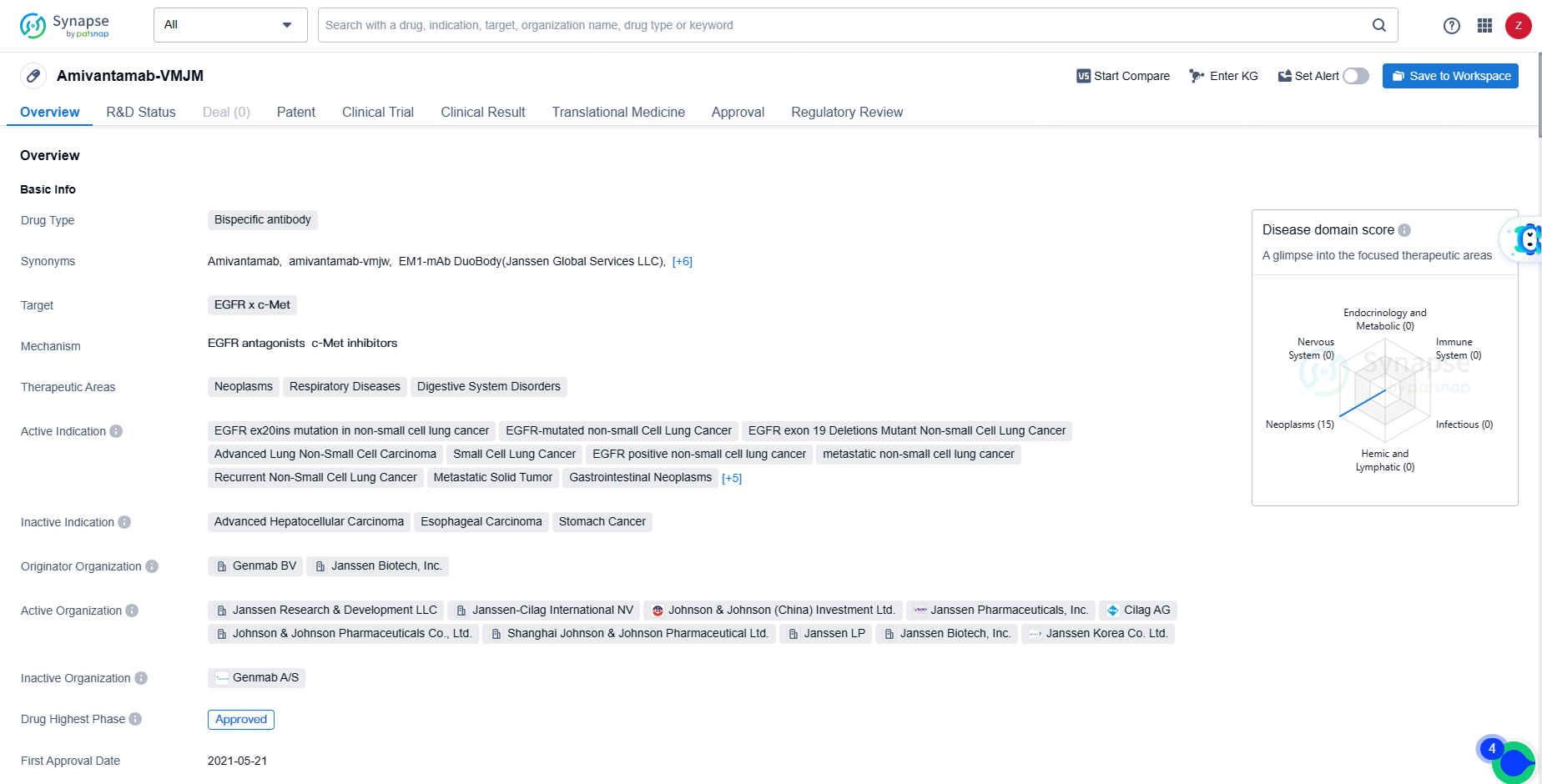
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Patients with EGFR-mutated advanced non-small-cell lung cancer who receive osimertinib treatment eventually experience resistance mechanisms and face suboptimal outcomes with platinum-based chemotherapy alone," stated Catherine Taylor, Vice President, EMEA Medical Affairs, Therapy Area Strategy at Janssen-Cilag AG. "Amivantamab targets a broad spectrum of EGFR and MET alterations, which are significant pathways of resistance to osimertinib. Combining amivantamab with chemotherapy has shown promise in overcoming resistance after osimertinib and extending disease control."
Amivantamab received conditional marketing authorisation from the European Commission in December 2021, making it the first fully-human, bispecific antibody approved for monotherapy in adult patients with advanced NSCLC harboring EGFR exon 20 insertion mutations following the failure of platinum-based chemotherapy.
The recent submission to the EMA is backed by data from the Phase 3 MARIPOSA-2 trial, which assessed the efficacy and safety of amivantamab combined with chemotherapy in patients with locally advanced or metastatic EGFR ex19del or L858R substitution NSCLC whose disease progressed on or after osimertinib treatment.
In the trial, the combination of amivantamab and chemotherapy achieved its primary endpoint by showing a statistically significant and clinically relevant improvement in progression-free survival compared to chemotherapy alone, cutting the risk of disease progression or death by 52%. The safety profile of amivantamab combined with chemotherapy was consistent with that of the individual components, and no new safety concerns emerged with the addition of amivantamab to chemotherapy.
The most frequent adverse events in the amivantamab arms included haematologic, EGFR, and MET-related events, with 58 percent of patients in the amivantamab plus chemotherapy group experiencing infusion-related reactions.
"The positive outcomes from the MARIPOSA-2 trial are the first to show a significant benefit in progression-free survival post-osimertinib," said Kiran Patel, M.D., Vice President, Clinical Development, Solid Tumors at Janssen Research & Development, LLC. "This highlights the potential of the amivantamab-chemotherapy combination in this patient population and underscores our commitment to improving patient outcomes."
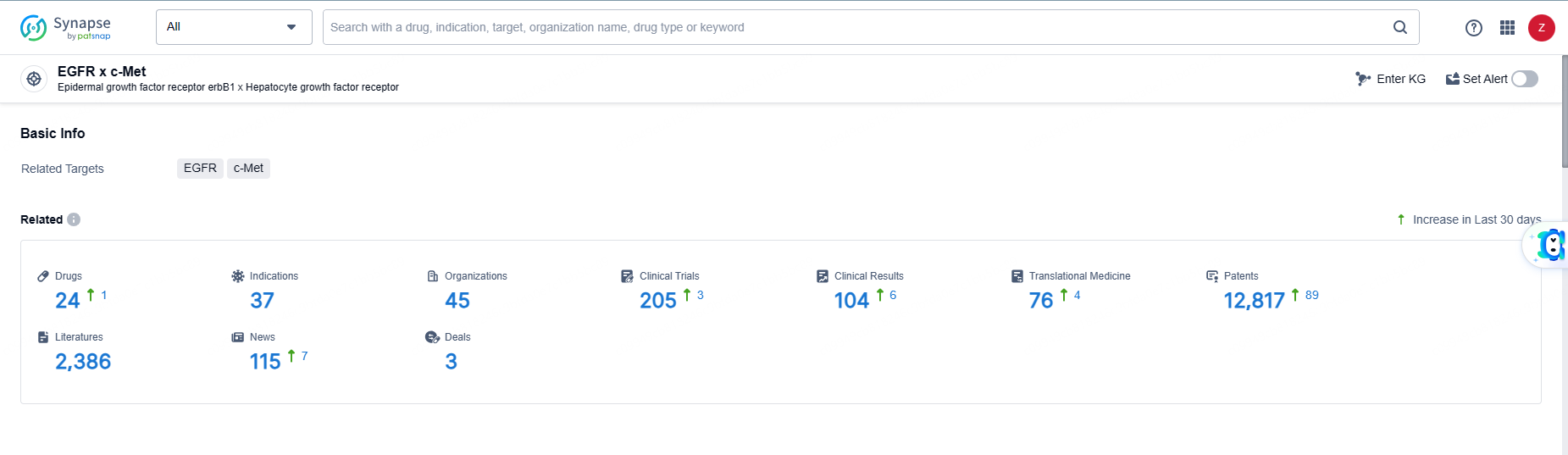
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 5, 2024, there are 24 investigational drugs for the EGFR-MET target, including 37 indications, 45 R&D institutions involved, with related clinical trials reaching 205, and as many as 12817 patents.
The approval of Amivantamab-VMJM represents a significant advancement in the treatment of various types of cancer, particularly non-small cell lung cancer with specific EGFR mutations. The drug's bispecific antibody nature allows it to target multiple proteins simultaneously, potentially leading to improved treatment outcomes for patients with these types of cancers.