Strand Therapeutics Announces Initial Patient Treated with STX-001 mRNA Therapy in Phase 1 Solid Tumor Study
Strand Therapeutics, recognized as the pioneering company in programmable mRNA technology focusing on treatments for cancer, autoimmune disorders, and other conditions, has declared that the initial patient has received a dosage in their Phase 1, first-in-human study of STX-001. This investigational treatment employs a multi-mechanistic, synthetic self-replicating mRNA platform to produce the IL-12 cytokine for a prolonged period within the tumor microenvironment.
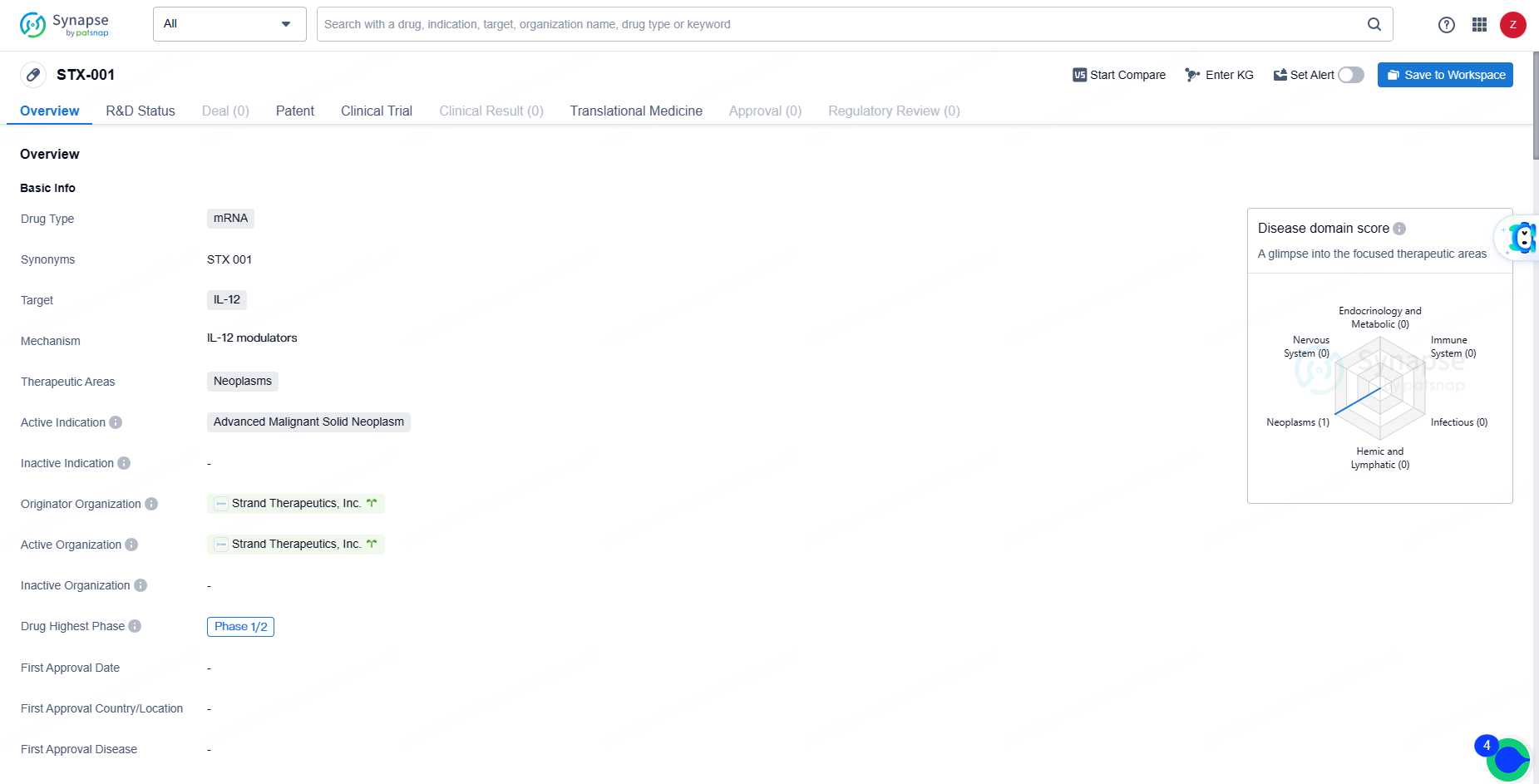
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"STX-001 marks the entry of the first programmable mRNA therapy in oncology into clinical trials for the treatment of solid tumors," stated Jake Becraft, Ph.D., CEO & Co-Founder of Strand Therapeutics. "We are immensely proud of this significant milestone for both patients and Strand Therapeutics, as we transition STX-001 from the laboratory to clinical evaluation, exploring the potential of this innovative cancer treatment for patients with solid tumors."
STX-001 has shown potential as an emerging method to enhance the effectiveness of immunotherapy for solid tumors. In preclinical studies using animal models, STX-001’s self-replicating mRNA technology triggered immunogenic death of cancer cells and facilitated the recruitment and activation of T cells and NK cells within the tumor microenvironment, through the direct expression of an IL-12 payload from synthetic mRNA within the tumor cells.
"Although current immunotherapies have made significant strides in addressing unmet patient needs across various solid tumors, further efforts are essential to achieve sustained clinical responses with minimal toxicities," noted Tasuku Kitada, Ph.D., Co-Founder, President, and Head of R&D at Strand Therapeutics. "We are excited to further our work with STX-001 and continue to lead the development of programmable mRNA therapies aimed at delivering precise immune activation within the tumor microenvironment."
The initial dosing of STX-001 in patients occurred just days before the upcoming American Society of Clinical Oncology Annual Meeting, slated for May 31 – June 4 in Chicago. Strand Therapeutics will display a poster detailing the design of the Phase 1 trial. This first-in-human, open-label, multi-center dose-escalation trial will assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary antitumor effects of STX-001, both as a monotherapy and in combination with pembrolizumab in patients with advanced solid tumors that are resistant to treatment.
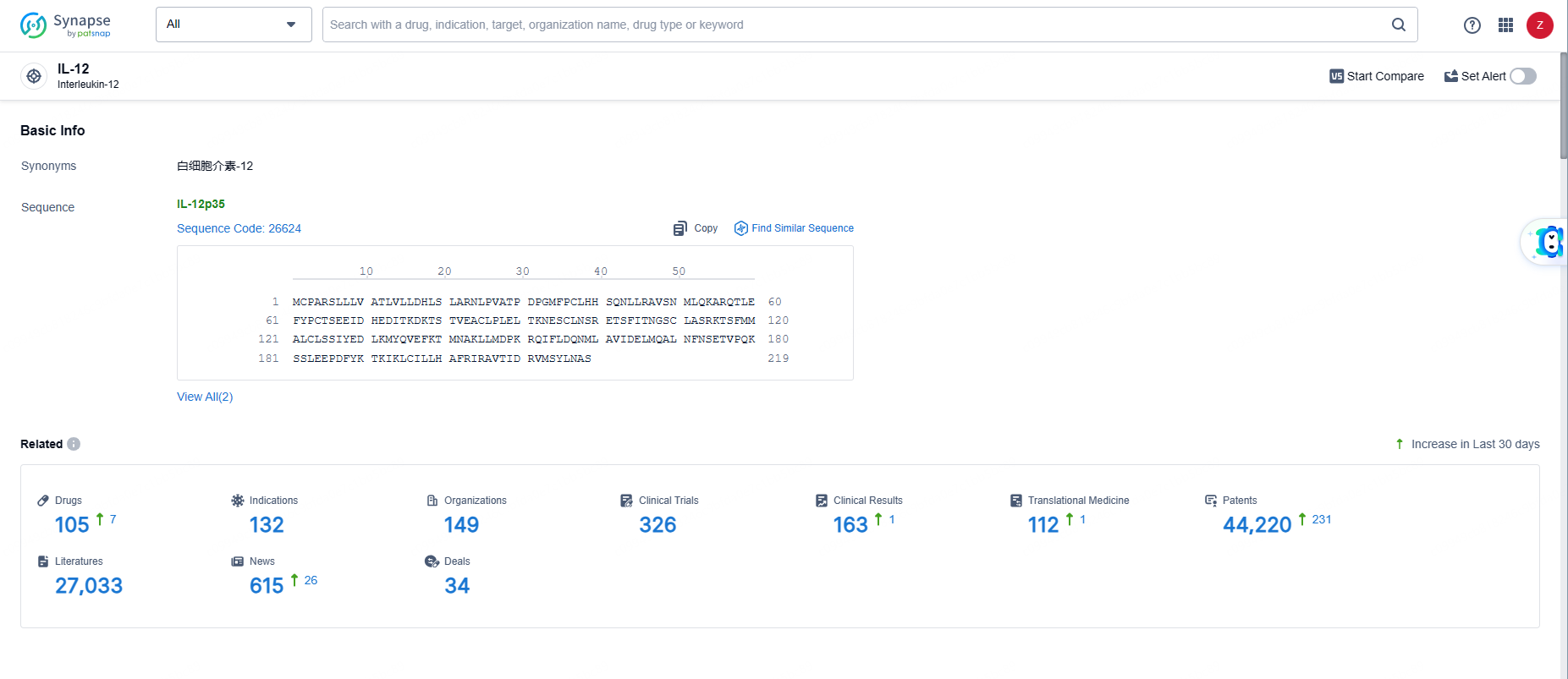
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 5, 2024, there are 105 investigational drugs for the IL-12 targets, including 132 indications, 149 R&D institutions involved, with related clinical trials reaching 326, and as many as 44220 patents.
STX-001 represents a novel mRNA drug targeting IL-12 for the treatment of advanced malignant solid neoplasms. Its advancement to the Phase 1/2 stage of clinical development indicates potential promise, but further research and development efforts will be required to determine its ultimate therapeutic value.