Research Progress and Future of KRAS G12C Inhibitors
The KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene is a GDP/GTP binding protein. KRAS is in the activated state when bound to GTP, and in the deactivated state when bound to GDP. KRAS can be briefly activated by growth factors or tyrosine kinases such as EGFR. The activated KRAS can then activate downstream pathways such as the PI3K-AKT-mTOR pathway, which controls cell generation, and the RAS-RAF-MEK-ERK pathway, which controls cell proliferation. Mutated KRAS becomes continuously activated, even in the absence of kinase activation such as EGFR, which leads to continuous cell proliferation and ultimately oncogenesis. Research has found that KRAS G12C is a specific sub-mutation of KRAS, where the 12th codon's glycine is replaced by cysteine. It accounts for about 44% of all KRAS mutations. Among them, it is most common in non-small cell lung adenocarcinomas (14%), followed by colorectal adenocarcinomas (3-4%), and pancreatic cancers (2%). Globally, more than 100,000 people are diagnosed with the KRAS G12C mutation each year.
KRAS G12C is a specific mutation of the KRAS gene that plays a crucial role in the development and progression of certain cancers. This mutation occurs in the KRAS protein, which is involved in regulating cell growth and division. In the human body, KRAS G12C mutation is commonly found in lung, colorectal, and pancreatic cancers. It leads to the activation of abnormal signaling pathways, promoting uncontrolled cell proliferation and tumor growth. Understanding the role of KRAS G12C is essential for developing targeted therapies that can specifically inhibit this mutation, offering potential treatment options for patients with KRAS G12C-mutated cancers.
KRAS G12C Competitive Landscape
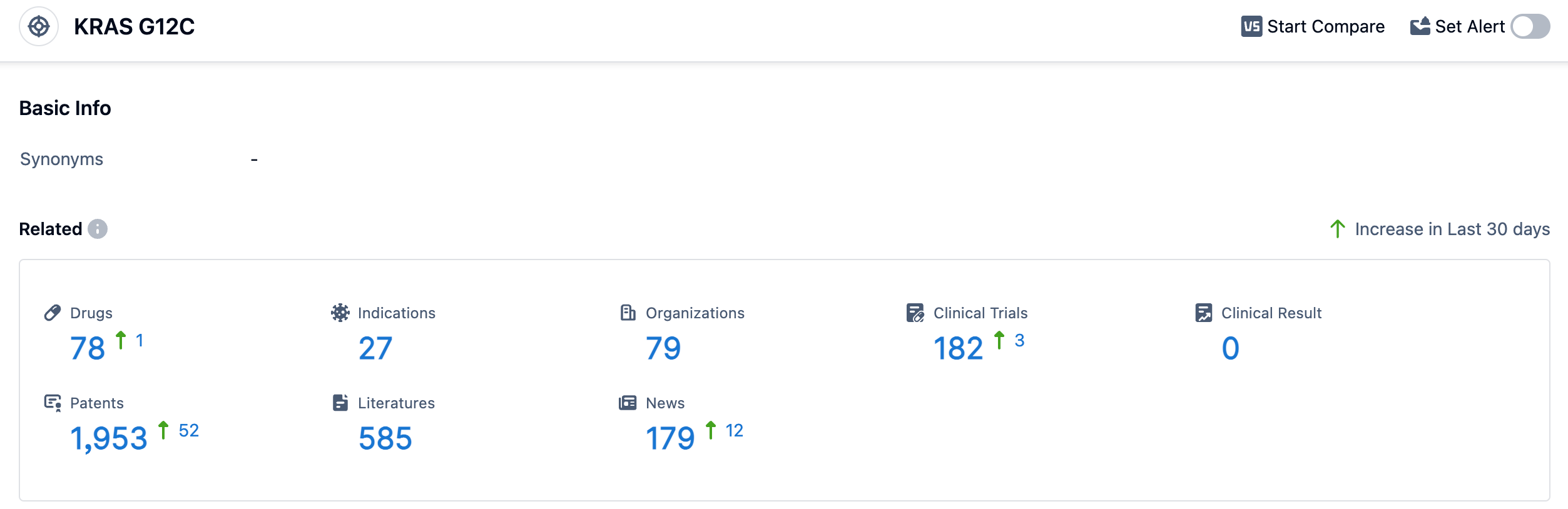
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 11 Sep 2023, there are a total of 78 KRAS G12C drugs worldwide, from 79 organizations, covering 27 indications, and conducting 182 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Several drugs under the target KRAS G12C have been approved for relevant indications. These indications include KRAS G12C mutant Non-small Cell Lung Cancer, Advanced Lung Non-Small Cell Carcinoma, metastatic non-small cell lung cancer, Colorectal Cancer, Endometrial Carcinoma, and Neoplasms. The drug types that are progressing most rapidly under the target KRAS G12C include small molecule drugs, PROTACs, oligonucleotides, TCR therapy, chemical drugs, biological products, small interfering RNA, and mRNA. Small molecule drugs have the highest number of drugs in approved, preclinical, and inactive stages. China has shown significant progress in the development of drugs targeting KRAS G12C. They have drugs in Phase 3, preclinical, and inactive stages. Other countries such as the United States, European Union, Japan, and the United Kingdom also have a considerable number of drugs in advanced stages of development.
Overall, the target KRAS G12C presents a competitive landscape with multiple companies and drug types involved in its development. The future development of this target holds promise for the treatment of various types of cancers, particularly those associated with KRAS G12C mutations.
Key drug:Sotorasib
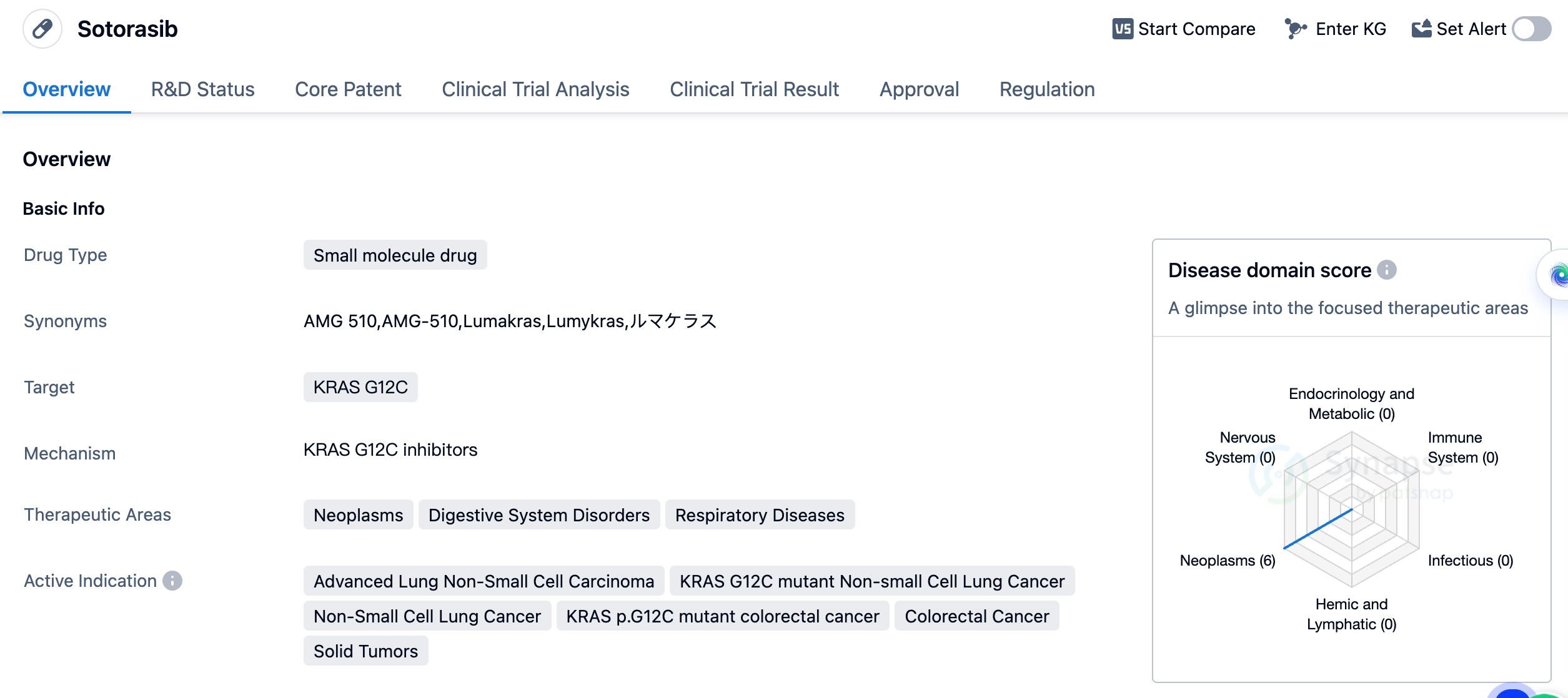
Sotorasib is a small molecule drug developed by Amgen, Inc. It falls under the category of biomedicine and is primarily used to target KRAS G12C, a specific mutation found in various types of cancers. The drug has shown potential therapeutic benefits in the treatment of neoplasms, digestive system disorders, and respiratory diseases. Sotorasib's active indications include advanced lung non-small cell carcinoma, KRAS G12C mutant non-small cell lung cancer, non-small cell lung cancer, KRAS p.G12C mutant colorectal cancer, colorectal cancer, and solid tumors. These indications highlight the drug's potential in treating a wide range of cancers, particularly those associated with the KRAS G12C mutation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Sotorasib received its first approval in the United States in May 2021, making it available for use in the American market. The drug's regulatory status includes accelerated approval, fast track designation, breakthrough therapy designation, and orphan drug designation. These regulatory designations highlight the potential significance and urgency of Sotorasib in addressing unmet medical needs and providing innovative treatment options for patients.
In summary, Sotorasib is a small molecule drug developed by Amgen, Inc. that specifically targets the KRAS G12C mutation. It has shown promise in treating various types of cancers, including advanced lung non-small cell carcinoma and colorectal cancer. With its recent approval in the United States and ongoing phase 3 development in China, Sotorasib represents a significant advancement in the field of biomedicine and offers potential therapeutic benefits for patients with specific genetic mutations.
Adagrasib
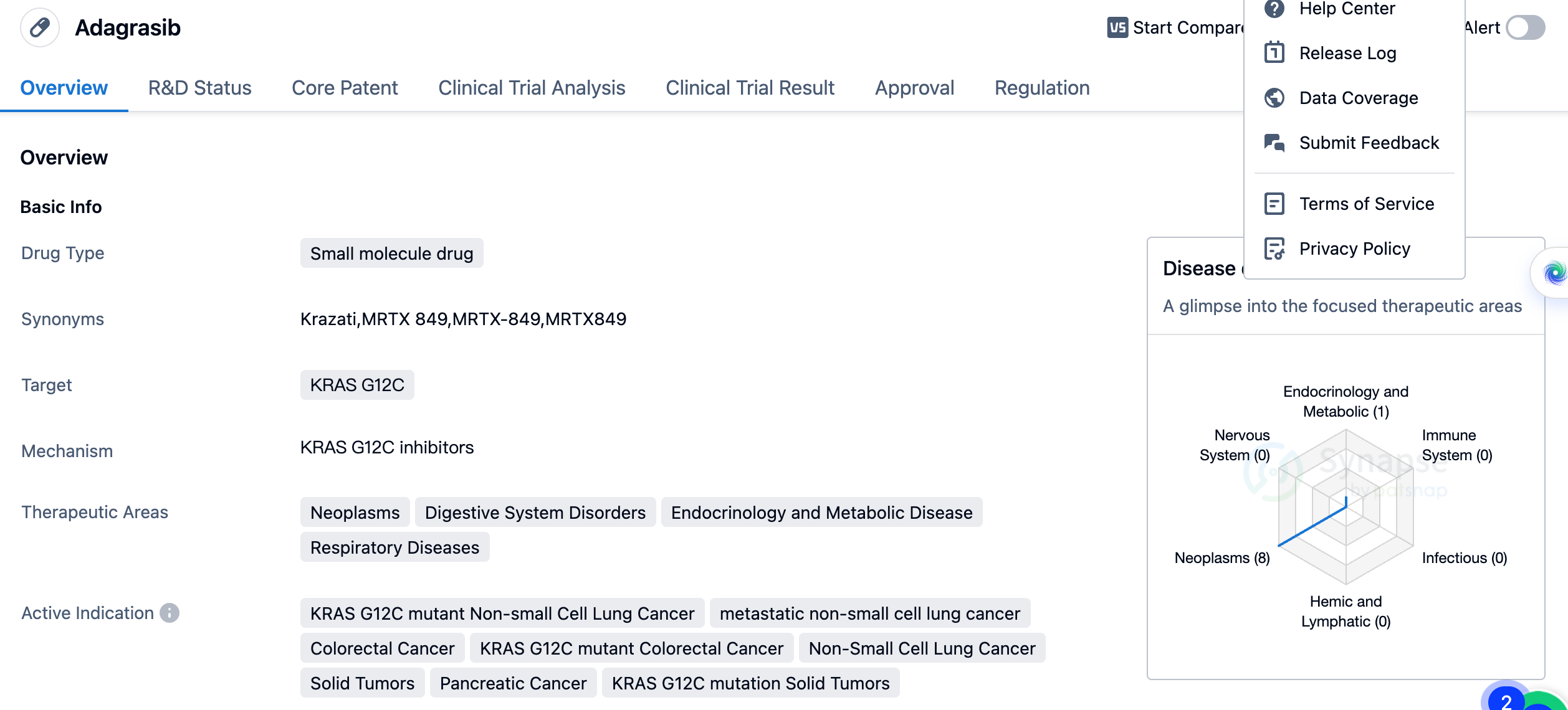
Adagrasib is a small molecule drug that targets the KRAS G12C mutation. It has shown potential therapeutic benefits in various therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic disease, and respiratory diseases. The drug is specifically indicated for KRAS G12C mutant non-small cell lung cancer, metastatic non-small cell lung cancer, colorectal cancer, KRAS G12C mutant colorectal cancer, non-small cell lung cancer, solid tumors, pancreatic cancer, and KRAS G12C mutation solid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The first approval of Adagrasib was granted in the United States, with a scheduled date of December 2022. The approval of Adagrasib is significant in the field of biomedicine, particularly in the treatment of cancers associated with the KRAS G12C mutation. This mutation is found in various types of cancer and has been challenging to target effectively. Adagrasib's approval offers new hope for patients with KRAS G12C mutant non-small cell lung cancer, colorectal cancer, and other solid tumors.
As a small molecule drug, Adagrasib has the potential for oral administration, which may provide convenience for patients compared to other treatment options. The drug's approval in the United States and ongoing phase 3 trials in China indicate its potential for global availability and impact.
In conclusion, Adagrasib is a small molecule drug developed by Array BioPharma, Inc. and Mirati Therapeutics, Inc. It targets the KRAS G12C mutation and has shown promise in various therapeutic areas, particularly in the treatment of cancers such as non-small cell lung cancer, colorectal cancer, and pancreatic cancer. The drug has received regulatory designations and is expected to receive its first approval in the United States in December 2022. Its approval offers new treatment options for patients with KRAS G12C mutant cancers and represents a significant development in the field of biomedicine.