MaaT Pharma Announces Phase 1b Success in ALS Treatment with MaaT033
MaaT Pharma, a biotechnology firm in the clinical development stage and a frontrunner in Microbiome Ecosystem TherapiesTM (MET) aimed at improving cancer patients' survival through immune modulation, has reported that the IASO trial (NCT05889572), an exploratory single-arm, open-label Phase 1b clinical study assessing MaaT033 in ALS, has achieved its primary objective of evaluating safety and tolerability with multiple dosing. The independent Data Safety and Monitoring Board (DSMB) has determined that the administration of MaaT033 over two months demonstrated favorable safety and tolerability in ALS patients. Initial microbiome evaluations indicate successful integration of MaaT033, the Company's oral capsule, further supporting the safety and tolerability findings.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Professor Gaëlle Bruneteau, a Neurology professor at Sorbonne University and a consultant neurologist at the Paris ALS expert center at Pitié-Salpêtrière Hospital in Paris, France, remarked, “I find the Phase 1b results promising as they highlight the robust safety and tolerability of MaaT033 in ALS. Both preclinical and clinical studies indicate the gut microbiota's involvement in ALS pathogenesis and its variability, making further research crucial to fully understand the gut-brain axis's role in this condition.”
Additional endpoints from the study are set to be evaluated in the upcoming months. The Data Safety Monitoring Board (DSMB) has endorsed advancing to Phase 2 based on available evidence from the Phase 1b IASO study. MaaT Pharma intends to decide on subsequent steps after a thorough review of the study's complete data, likely available in early 2025. This may include launching a larger randomized controlled efficacy trial, contingent upon securing appropriate funding.
Hervé Affagard, CEO and co-founder of MaaT Pharma, expressed his deep appreciation to the patients enrolled in this study while facing a severe illness. He stated, “The ALS trial marks a potentially groundbreaking milestone in our efforts to enhance patient survival through innovative therapies based on microbiome-mediated immune modulation. The findings indicate the potential flexibility of our platform to meet significant unmet medical needs across various therapeutic fields. As we aim to broaden the reach and effectiveness of this innovation, we will actively seek collaboration opportunities to expedite and extend its applications to assist more patients in need.”
Fifteen participants from two centers in France have taken part in the Phase 1 trial. This research has been a joint initiative involving prominent researchers, clinicians from Hôpital de la Pitié-Salpêtrière-AP-HP, and University Hospital of Lille, as well as specialists from the French academic FILSLAN/ACT4ALS-MND and the French patients’ association Tous en Selles contre la SLA. These findings, along with earlier results from the Phase 1b CIMON trial in Acute Myeloid Leukemia and the recent DSMB feedback on the ongoing Phase 2b trial PHOEBUS in Europe, strengthen confidence in the safety profile of MaaT033 for continued use.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
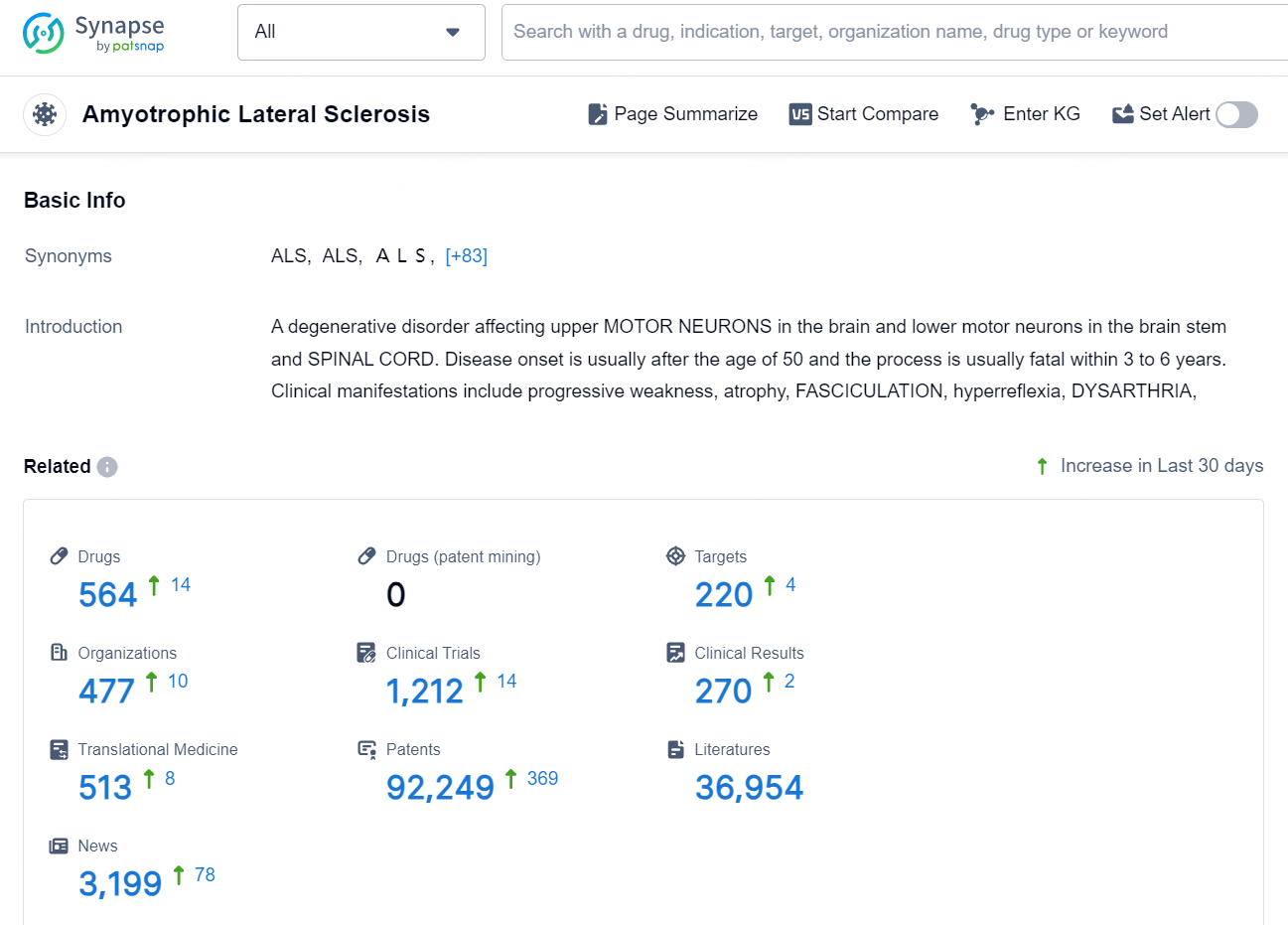
According to the data provided by the Synapse Database, As of December 2, 2024, there are 564 investigational drugs for the Amyotrophic Lateral Sclerosis, including 220 targets, 477 R&D institutions involved, with related clinical trials reaching 1212, and as many as 92249 patents..
MaaT-033 is a live biotherapeutic product developed by Maat Pharma SA, designed to target a wide range of therapeutic areas including Immune System Diseases, Infectious Diseases, Skin and Musculoskeletal Diseases, Neoplasms, Nervous System Diseases, Endocrinology and Metabolic Disease, Hemic and Lymphatic Diseases, and other diseases.