Korro Initiates First Human Study of KRRO-110, Forms Clinical Advisory Board
Korro Bio, Inc. (Nasdaq: KRRO), a biopharmaceutical firm dedicated to creating an innovative category of genetic therapies through RNA editing for both uncommon and widespread diseases, has announced that it has submitted an application to the Bellberry HREC for a Phase 1/2 clinical trial of KRRO-110 targeting AATD. Additionally, a Clinical Advisory Board (CAB), consisting of renowned researchers and specialists in lung and liver conditions related to AATD, has been formed to provide guidance on the clinical development strategy for KRRO-110.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“This regulatory submission signifies Korro’s shift into a clinical-stage organization and marks another crucial milestone in the advancement of KRRO-110,” stated Kemi Olugemo, MD, Chief Medical Officer at Korro. “There is a considerable unmet medical requirement for individuals affected by AATD. Our preclinical findings indicate that KRRO-110 may represent a leading therapy, reflecting our unique approach to RNA editing. The extensive pulmonary and hepatic expertise within our CAB will play a critical role in shaping our clinical and regulatory strategy, ensuring we address the comprehensive needs of AATD patients. We are privileged to collaborate with the foremost experts in AATD who share our dedication to scientific rigor and enhancing patient outcomes.”
Pending approval from the HREC and the acceptance of the clinical trial notification (CTN) by Australia’s Therapeutic Goods Administration (TGA), Korro expects to initiate dosing for the first participant in the Phase 1/2 study during the first quarter of 2025. An interim analysis of data from the Phase 1/2 trial of KRRO-110 is projected for the second half of 2025, with the study's completion anticipated in 2026.
“This represents a significant milestone for a company rooted in RNA editing, transitioning from early concepts to the initiation of a first-in-human clinical trial. I take great pride in the advancements we've achieved since the nomination of KRRO-110 for AATD in December 2023, as well as the efforts of our entire Korro team,” added Ram Aiyar, PhD, CEO and President of Korro. “Our strong pipeline, consisting of fully owned and partnered initiatives, highlights the transformative capabilities of our OPERA™ platform. With a robust financial position to facilitate the completion of the Phase 1/2 study and to advance our subsequent programs, I am eager to witness the potential of our platform come to fruition.”
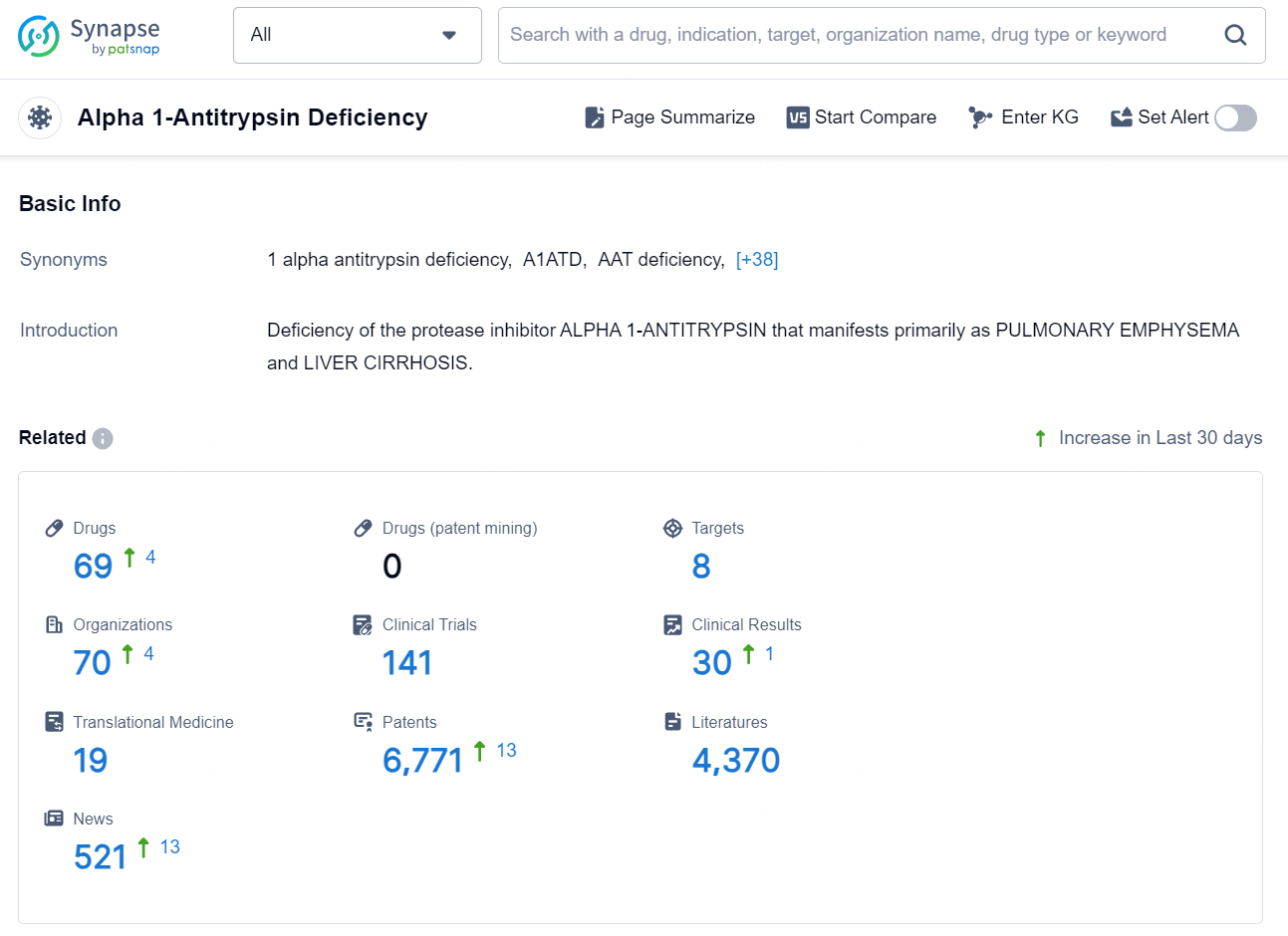
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 7, 2024, there are 69 investigational drugs for the Alpha 1-Antitrypsin Deficiency, including 8 targets, 70 R&D institutions involved, with related clinical trials reaching 141, and as many as 6771 patents.
AATD is a genetic disorder caused by a single missense mutation (G-to-A) in the SERPINA1 gene. Affected adult individuals experience pulmonary emphysema and/or hepatic cirrhosis, as well as end organ manifestations. KRRO-110 is the first RNA editing oligonucleotide product candidate from Korro’s proprietary RNA editing platform, Oligonucleotide Promoted Editing of RNA (OPERA™). KRRO-110 is designed to co-opt an endogenous enzyme, Adenosine Deaminase Acting on RNA’s (ADAR), to edit the “A” variant on SERPINA1 RNA, repair an amino acid codon, and restore secretion of normal AAT protein. This repair of the endogenous protein has the potential to clear protein aggregates from within liver cells to create a potentially clinically differentiated benefit for liver function and to preserve lung function by providing an adequate amount of normal AAT protein.