Latest Developments in the Research and Development of BET inhibitor
BET (Bromodomain and Extra-Terminal) proteins play a crucial role in the regulation of gene expression in the human body. These proteins are involved in the recognition and binding of acetylated lysine residues on histone proteins, which are responsible for controlling gene transcription. By binding to these acetylated lysine residues, BET proteins facilitate the recruitment of transcriptional machinery and other regulatory proteins to specific gene loci, thereby influencing gene expression. This makes BET proteins important regulators of various cellular processes, including cell growth, differentiation, and inflammation. Due to their involvement in gene regulation, BET proteins have emerged as potential therapeutic targets for various diseases, including cancer and inflammatory disorders.
BET Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 60 BET drugs worldwide, from 62 organizations, covering 64 indications, and conducting 83 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.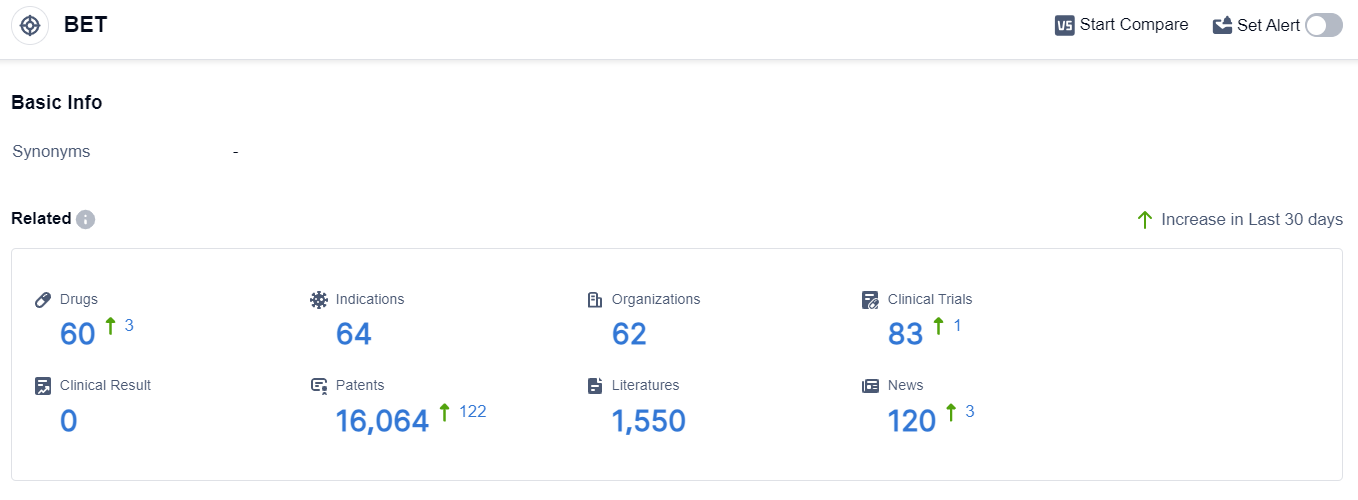
The current competitive landscape of target BET is characterized by the presence of multiple companies at different stages of development. MorphoSys AG is leading with drugs in Phase 3 and Phase 2. Bristol Myers Squibb Co., GSK Plc, Zenith Epigenetics Ltd., and other companies also have drugs in advanced stages of development.
The indications for drugs under target BET cover a wide range of diseases, including various types of cancers. Small molecule drugs, AAV based gene therapy, PROTACs, and other drug types are being researched and developed for target BET.
The development of target BET is progressing in countries such as the United States, Canada, European Union, Spain, United Kingdom, and China. China has shown progress in the development of drugs for target BET.
The future development of target BET is expected to witness intense competition, especially in the small molecule drug category, as indicated by the presence of biosimilars. Further research and development by pharmaceutical companies and institutions will contribute to the advancement of treatments for diseases associated with target BET.
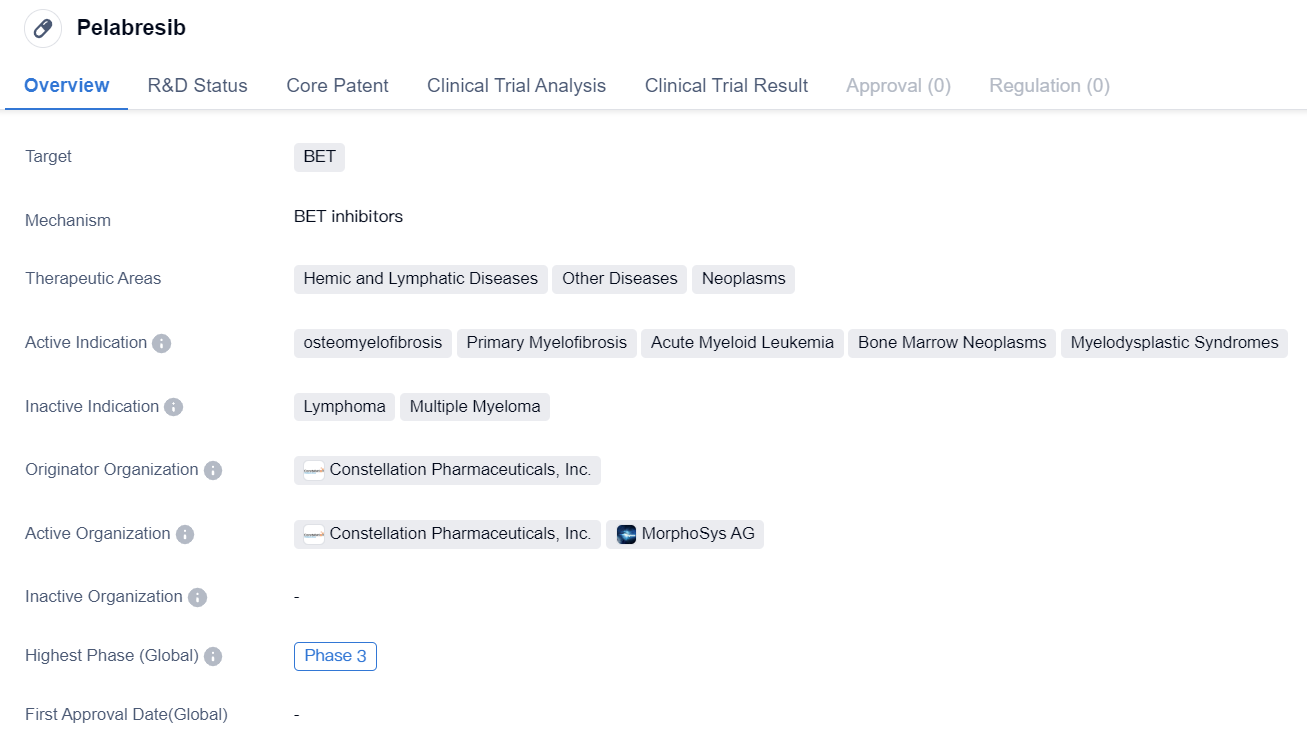
BET Inhibitor in Phase III Clinical Trials: Pelabresib
Pelabresib is a small molecule drug that falls under the category of biomedicine. It specifically targets BET proteins, which are involved in various cellular processes and have been implicated in the development and progression of several diseases. The drug is being developed by Constellation Pharmaceuticals, Inc., a pharmaceutical company specializing in the field of epigenetics.
Pelabresib has shown potential therapeutic benefits in the treatment of various diseases, particularly those related to the hemic and lymphatic systems. It has demonstrated efficacy in the treatment of osteomyelofibrosis, a rare bone marrow disorder characterized by the replacement of bone marrow with fibrous tissue. The drug has also shown promise in the treatment of primary myelofibrosis, a chronic blood cancer that affects the bone marrow and leads to the formation of scar tissue.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In addition to these indications, pelabresib has shown activity against acute myeloid leukemia, a type of cancer that affects the blood and bone marrow. It has also shown potential in the treatment of bone marrow neoplasms, which are abnormal growths or tumors that develop in the bone marrow. Furthermore, the drug has demonstrated efficacy in myelodysplastic syndromes, a group of disorders characterized by abnormal production of blood cells in the bone marrow.
Currently, pelabresib has reached Phase 3 of clinical development. This phase involves large-scale clinical trials to evaluate the safety and efficacy of the drug in a larger patient population. The results from these trials will provide crucial data to support the potential approval and commercialization of pelabresib.
In summary, pelabresib is a small molecule drug developed by Constellation Pharmaceuticals, Inc. It targets BET proteins and has shown potential therapeutic benefits in the treatment of various diseases, including osteomyelofibrosis, primary myelofibrosis, acute myeloid leukemia, bone marrow neoplasms, and myelodysplastic syndromes. The drug is currently in Phase 3 of clinical development, and further studies will determine its safety and efficacy for potential approval and commercialization.
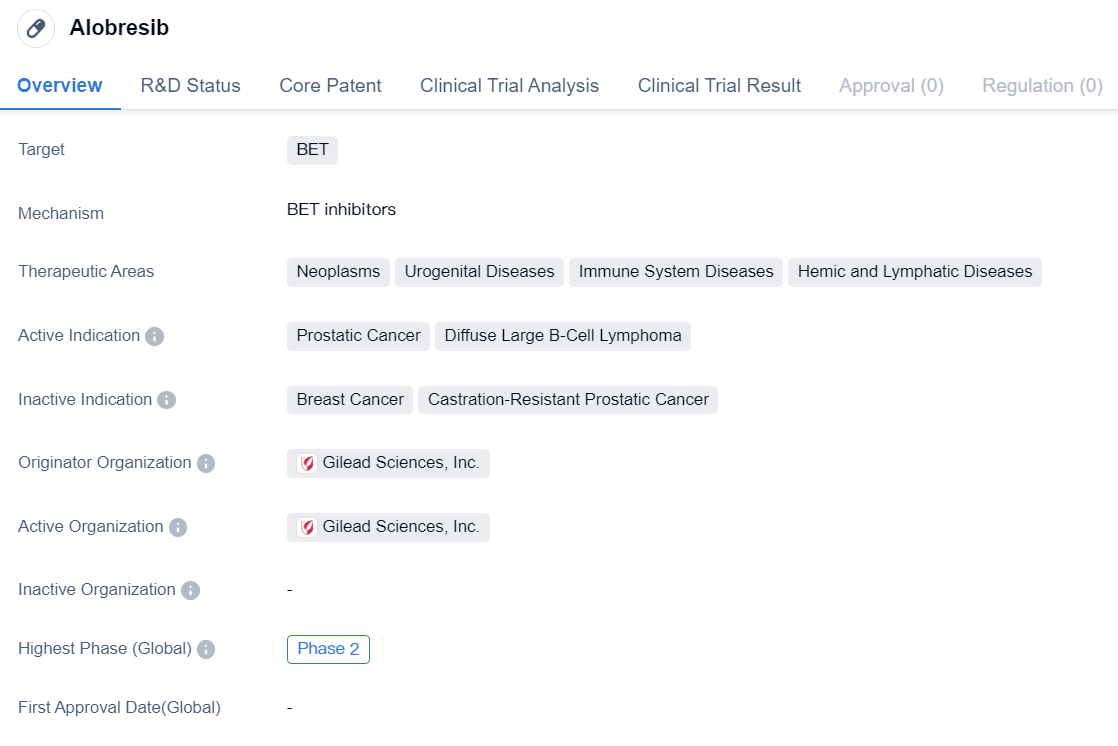
BET inhibitor entering clinical phase II: Alobresib
Alobresib is a small molecule drug that falls under the category of biomedicine. It specifically targets BET, which stands for bromodomain and extra-terminal proteins. This drug has shown potential in treating various therapeutic areas including neoplasms, urogenital diseases, immune system diseases, and hemic and lymphatic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of active indications, Alobresib has demonstrated efficacy in the treatment of prostatic cancer and diffuse large B-cell lymphoma. These are both serious and potentially life-threatening conditions, making the development of effective treatments crucial.
The originator organization of Alobresib is Gilead Sciences, Inc. Gilead Sciences is a renowned pharmaceutical company known for its contributions to the field of biomedicine. As the originator organization, Gilead Sciences is responsible for the initial discovery and development of Alobresib.
Currently, Alobresib has reached Phase 2 of clinical development. Clinical trials are an essential part of drug development, as they help determine the safety and efficacy of a drug in a controlled setting. Phase 2 trials involve a larger number of participants and aim to further evaluate the drug's effectiveness and side effects.
In summary, Alobresib is a small molecule drug that targets BET and shows promise in treating various therapeutic areas such as neoplasms, urogenital diseases, immune system diseases, and hemic and lymphatic diseases. It is actively being studied for its potential in treating prostatic cancer and diffuse large B-cell lymphoma. Gilead Sciences, Inc. is the originator organization behind the development of Alobresib. Currently, the drug has reached Phase 2 of clinical trials, indicating progress in its development and potential for future use in patients.