Mozart Therapeutics presents MTX-101 trial outcomes, a dual-action CD8 Treg modifier for autoimmune treatment, at 2023 ACR Convergence
At the American College of Rheumatology’s annual gathering at the San Diego Convention Center in San Diego, CA, Mozart Therapeutics, a renowned developer of CD8 Treg Modulators utilized in combating autoimmune and inflammatory diseases, showcased preclinical pharmacological data and tolerability analytics for MTX-101, a CD8 Treg modulator.
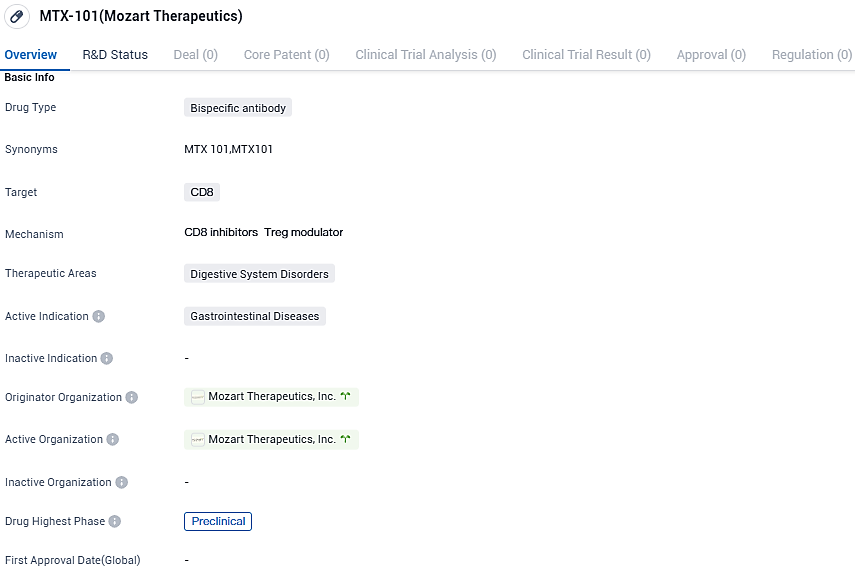
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
CD8 Treg can be found in rheumatological autoimmune disorders, exhibiting a diminished expression of functional proteins and a decreased reaction to stimulation compared to CD8 Treg from healthy donors. By specifically binding to CD8 Treg, MTX-101 promotes their ability to eliminate self-reactive CD4 T cells through the obstruction of inhibitory KIR.
This heightening of the MTX-101 facilitated CD8 Treg function restrains the proliferation of self-reactive CD4 T cells and inflammation, with no unintentional activation of immune cells nor a rise in pro-inflammatory cytokines.
In non-clinical safety evaluations, doses of up to 50 mg/kg of MTX-101 were well-received. "These findings are critical as they demonstrate the therapeutic efficacy of MTX-101, corroborating the capacity of MTX-101 to rejuvenate CD8 Treg functionality in autoimmune diseases and to bring immune balance back," stated Kristine Swiderek, PhD, the Chief Scientific Officer at Mozart Therapeutics.
Serving as a bispecific CD8 Treg Modulator designed for inhibitory KIR and CD8 found on regulatory CD8 T cells, MTX-101 is an autoimmune checkpoint inhibitor that aspires to mend the operation of regulatory CD8 T cells, taking action at the inception of the autoimmune disease cycle to stop downstream inflammation and cease the continuation of tissue damage.
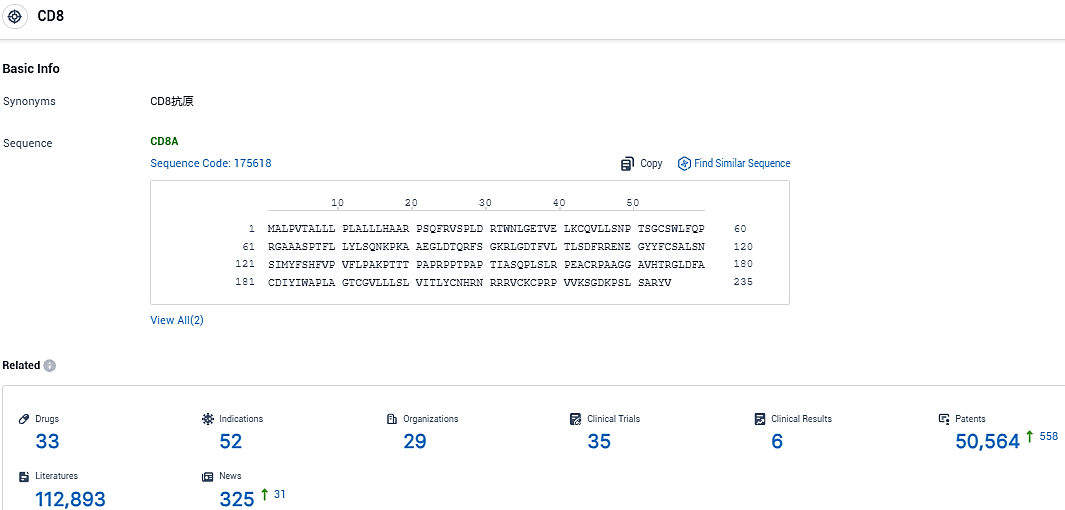
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 21, 2023, there are 33 investigational drugs for the CD8 target, including 52 indications, 29 R&D institutions involved, with related clinical trials reaching 35, and as many as 50564 patents.
MTX-101 targets CD8 and aims to address gastrointestinal diseases. Currently in the preclinical phase, MTX-101 holds promise for the future treatment of digestive system disorders, but further research and clinical trials are needed to determine its safety and efficacy in humans.