SCG Cell Therapy finds that SCG101 boosts tumor response and sustains antiviral effects in advanced liver cancer patients with HBV
Late clinical results were unveiled by SCG Cell Therapy Pte Ltd,, a biotechnological firm in the clinical phase developing fresh immunotherapies against infectious diseases and related cancers, at the 2023 AASLD Liver Meeting in Boston, USA. This groundbreaking data derives from SCG101, their pioneering autologous HBV-specific T-cell receptor-engineered T Cell treatment - a first in its class.
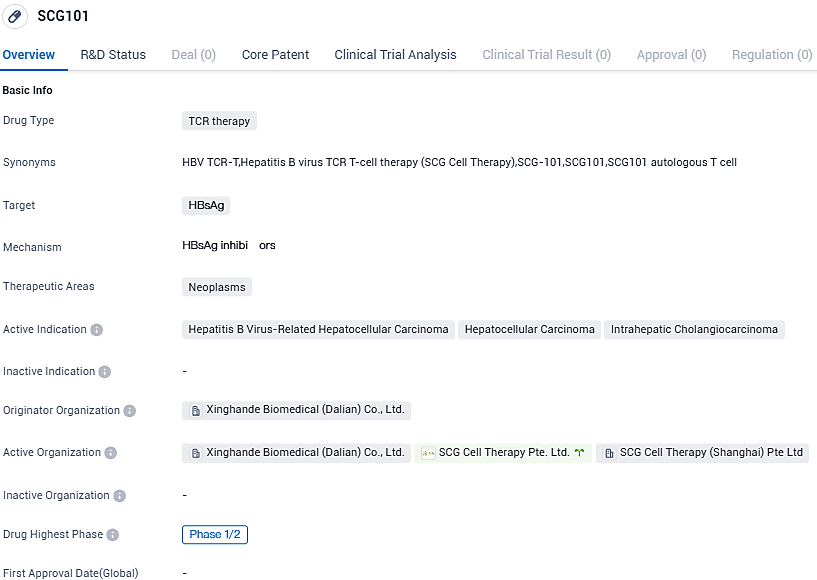
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Clinical trial data from the first human trials reveals promising antiviral and antitumor activity in advanced HBV-related hepatocellular carcinoma patients using SCG101. Out of six patients who received a single IV dose of SCG101 at 5.0×107 ~ 1.0×108 TCR+ T cells/kg, there were partial responses in two patients, and two patients saw stabilizing conditions with observable tumor reduction.
Strong correlations were observed between the antiviral activity of SCG101 and the tumor responses. As per the cutoff data, serum HBsAg reduction was experienced by all six patients, with four achieving a reduction of 1~3 log post-SCG101 infusion.
The safety evaluation showed that SCG101 was generally well tolerated, with no serious incidents of adverse effects or neurotoxicity syndrome linked to immune effector cells. The adverse effects related to the treatment, such as transient liver enzymes evaluation, cytokine release syndrome, and fever, were anticipated due to the working mechanism of SCG101 on diseased hepatocytes clearance and immune activation.
SCG101 has the ability to precisely target an HBV peptide presented on HBV-HCC tumor cells, premalignant hepatocytes integrated with HBV-DNA and cells infected with HBV, thereby initiating cytolytic and non-cytolytic methods to eradicate tumor cells and HBV-infected cells.
Frank Wang, the CEO of SCG Cell Therapy stated, "We are thrilled to lead in illustrating the potential of HBV TCR T cell therapy in obtaining promising tumor responses in solid tumors, as well as inducing lasting antiviral activity. This distinctive double function of SCG101 introduces a fresh approach to treat HBV-associated HCC by targeting the root cause of the cancer."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 21, 2023, there are 38 investigational drugs for the HBsAg target, including 14 indications, 42 R&D institutions involved, with related clinical trials reaching 225, and as many as 6706 patents.
SCG101, an autologous T-cell receptor T cell therapy, is an investigational cell therapy product targeting a specific epitope of hepatitis B surface antigen (HBsAg). SCG101 was granted clinical trial approvals by FDA, NMPA and Singapore Health Science Authority and Hong Kong Department of Health for patients with HBV-related HCC.