Latigo Biotherapeutics Initiates Phase 1 Trial of LTG-305 for Non-Opioid Pain Management
Latigo Biotherapeutics, Inc. has announced that dosing has commenced for the first participant in its Phase 1 clinical trial of LTG-305, which is being investigated as a leading non-opioid treatment option for chronic pain. The Phase 1 trial (NCT06554574) aims to assess the safety, tolerability, and pharmacokinetics (PK) of LTG-305 in healthy subjects, utilizing both single-ascending dose (SAD) and multiple-ascending dose (MAD) cohorts.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
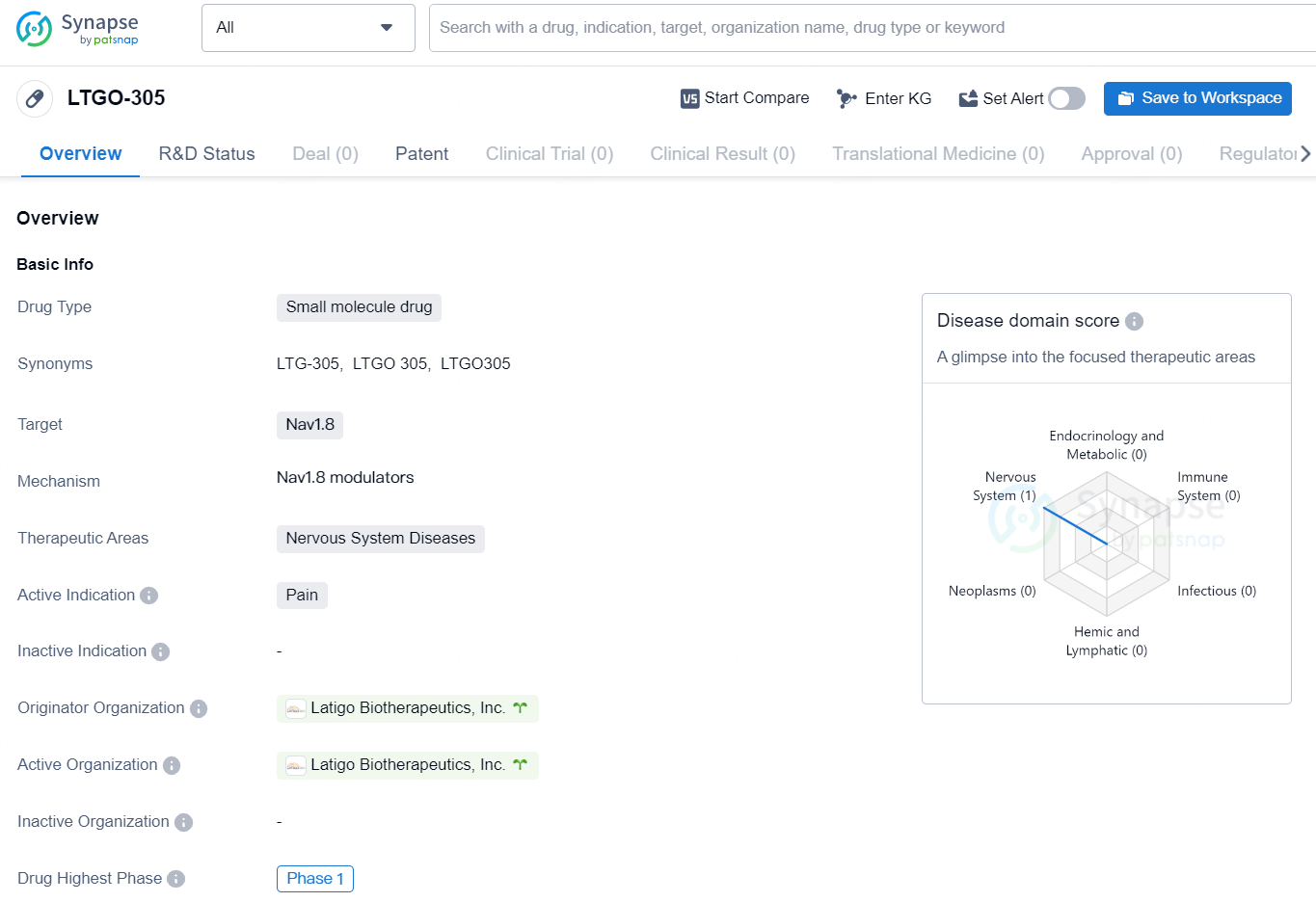
LTG-305 is an inhibitor of Nav1.8 specifically created to address the fundamental causes of pain directly at the source. This innovative small molecule presents a promising non-opioid strategy for chronic pain management, offering a safer and more precise treatment alternative for patients. Nav1.8, which is a voltage-gated sodium channel, is essential for the transmission of pain sensations. By targeting Nav1.8 selectively, LTG-305 seeks to deliver effective pain relief while minimizing central nervous system side effects commonly seen with traditional therapies, such as addiction, dizziness, and drowsiness.
“This significant achievement in the clinical advancement of LTG-305 adds another potential leading Nav1.8 inhibitor to our range of non-opioid pain management options in development,” stated Neil Singla, M.D., chief medical officer of Latigo Biotherapeutics. “We have recently reported positive results from the Phase 1 trial of our candidate, LTG-001, aimed at acute pain, and moving LTG-305 into clinical trials showcases our belief in the potential of Nav1.8 inhibitors for both acute and chronic pain treatment.”
The Phase 1 trial will evaluate LTG-305 in healthy subjects across various dosing levels, focusing primarily on assessing its safety and tolerability. Latigo expects to present the topline results from this trial by mid-2025.
During the Single Ascending Dose (SAD) segment of the trial, healthy participants will receive a single administration of LTG-305 at escalating doses to evaluate its safety, tolerability, and pharmacokinetics (PK). Following the SAD phase, the Multiple Ascending Dose (MAD) segment will have participants taking multiple doses of LTG-305 over a specified timeframe.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
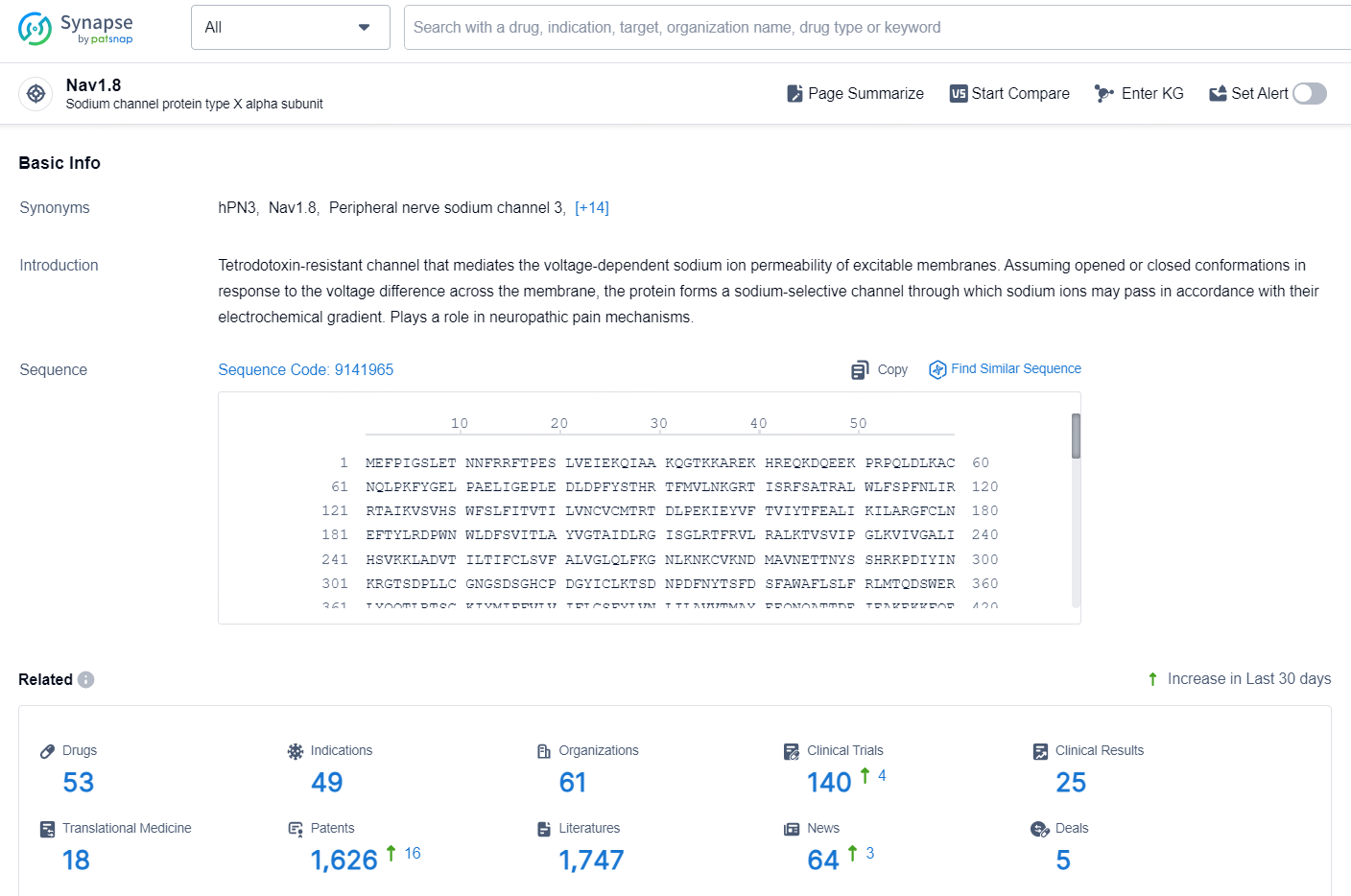
According to the data provided by the Synapse Chemical, As of October 21, 2024, there are 53 investigational drugs for the Nav1.8 target, including 49 indications, 61 R&D institutions involved, with related clinical trials reaching 140, and as many as 1626 patents.
LTGO-305 is a small molecule drug developed by Latigo Biotherapeutics, Inc., targeting Nav1.8 as a potential treatment for pain in the therapeutic area of Nervous System Diseases. The drug is currently in Phase 1, the highest phase of clinical development globally.