Lilly's Tirzepatide Cuts Heart Failure Risk by 38% in HFpEF Patients with Obesity
UCB (Euronext Brussels: UCB) alongside Biogen Inc. (NASDAQ: BIIB) disclosed comprehensive findings from the Phase 3 PHOENYCS GO trial, which assessed dapirolizumab pegol (DZP), an innovative Fc-free anti-CD40L therapeutic candidate. This trial showcased substantial clinical enhancements in disease activity among patients suffering from moderate to severe systemic lupus erythematosus (SLE). The findings were presented during a late-breaking oral session at ACR Convergence 2024, the annual meeting of the American College of Rheumatology, held in Washington, DC.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
There is still a notable need for additional treatment alternatives for individuals diagnosed with systemic lupus erythematosus. The outcomes observed in the PHOENYCS GO study indicate that dapirolizumab pegol could play a significant role in managing this chronic and debilitating autoimmune condition. "Our findings across various clinical endpoints demonstrated a positive impact and an encouraging safety profile," stated Megan E.B. Clowse, M.D., the study's principal investigator and Associate Professor of Medicine, who leads the Division of Rheumatology and Immunology at Duke University School of Medicine. "Participants treated with dapirolizumab pegol showed a decrease in lupus activity while successfully reducing steroid use, which is a vital aspect for those living with the condition."
In the PHOENYCS GO trial (n=321), dapirolizumab pegol (DZP) was given intravenously every four weeks. For the primary endpoint, which evaluated the improvement in moderate-to-severe disease activity via the British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment (BICLA) after a 48-week period, those receiving DZP along with standard of care (SOC) achieved a statistically significant 14.6% higher response rate (49.5%) compared to those treated with SOC alone (34.6%) (95% confidence interval [CI]: 3.3, 25.8; p=0.0110). A higher BICLA response rate suggests an effective treatment impact across all affected organs at baseline, correlating with meaningful clinical outcomes.
For the first secondary endpoint assessing the BICLA response at Week 24, participants receiving DZP with SOC had a 7.9% higher response rate (46.6%) compared to those on SOC alone (38.3%). However, this difference did not achieve statistical significance (95% CI: -3.6, 19.4; p=0.1776). Due to the lack of statistical significance for this initial key secondary endpoint in the hierarchical testing, all subsequent secondary endpoint analyses are described without statistical significance, providing nominal p-values.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
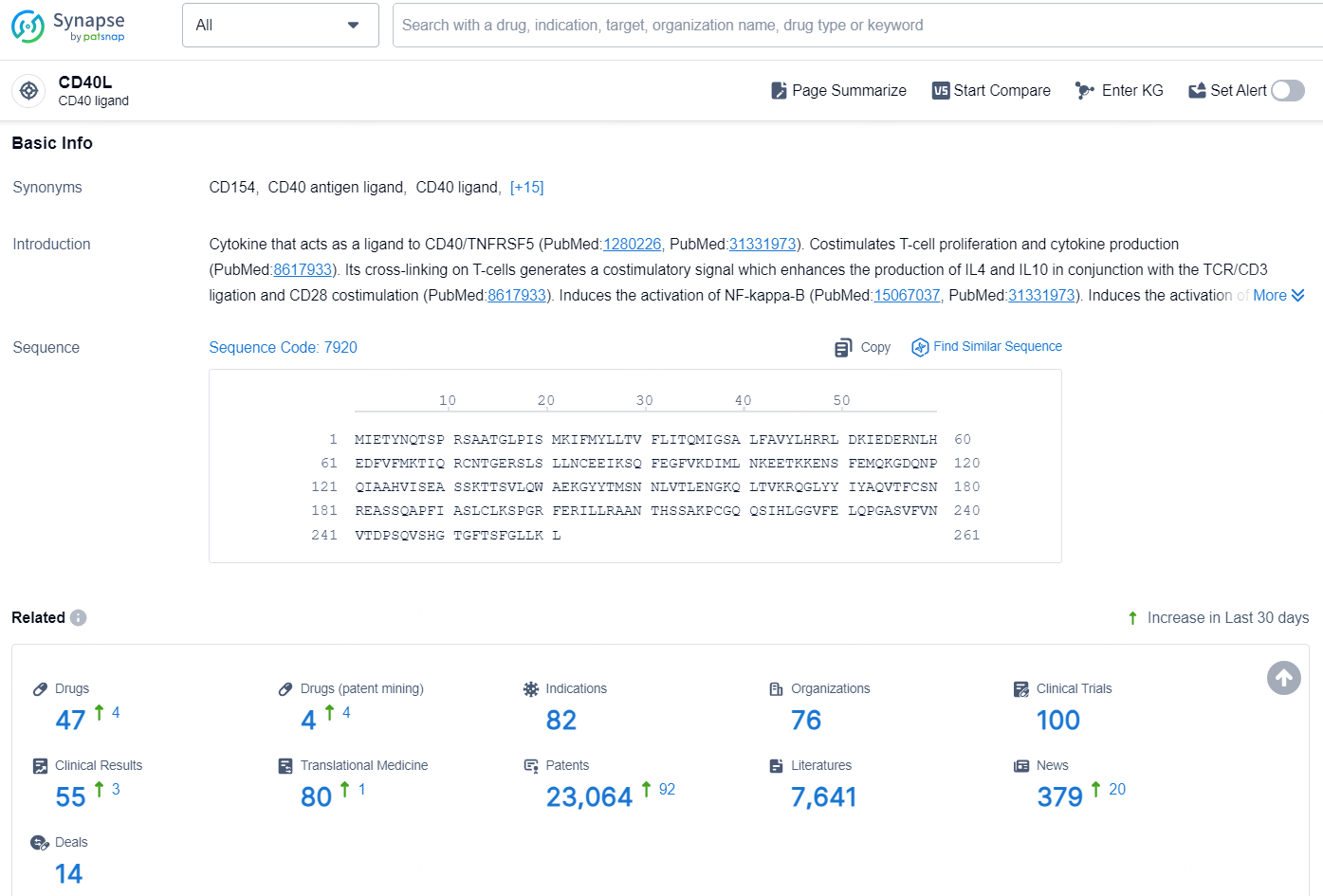
According to the data provided by the Synapse Database, As of November 25, 2024, there are 47 investigational drugs for the CD40L target, including 82 indications, 76 R&D institutions involved, with related clinical trials reaching 100, and as many as 23064 patents.
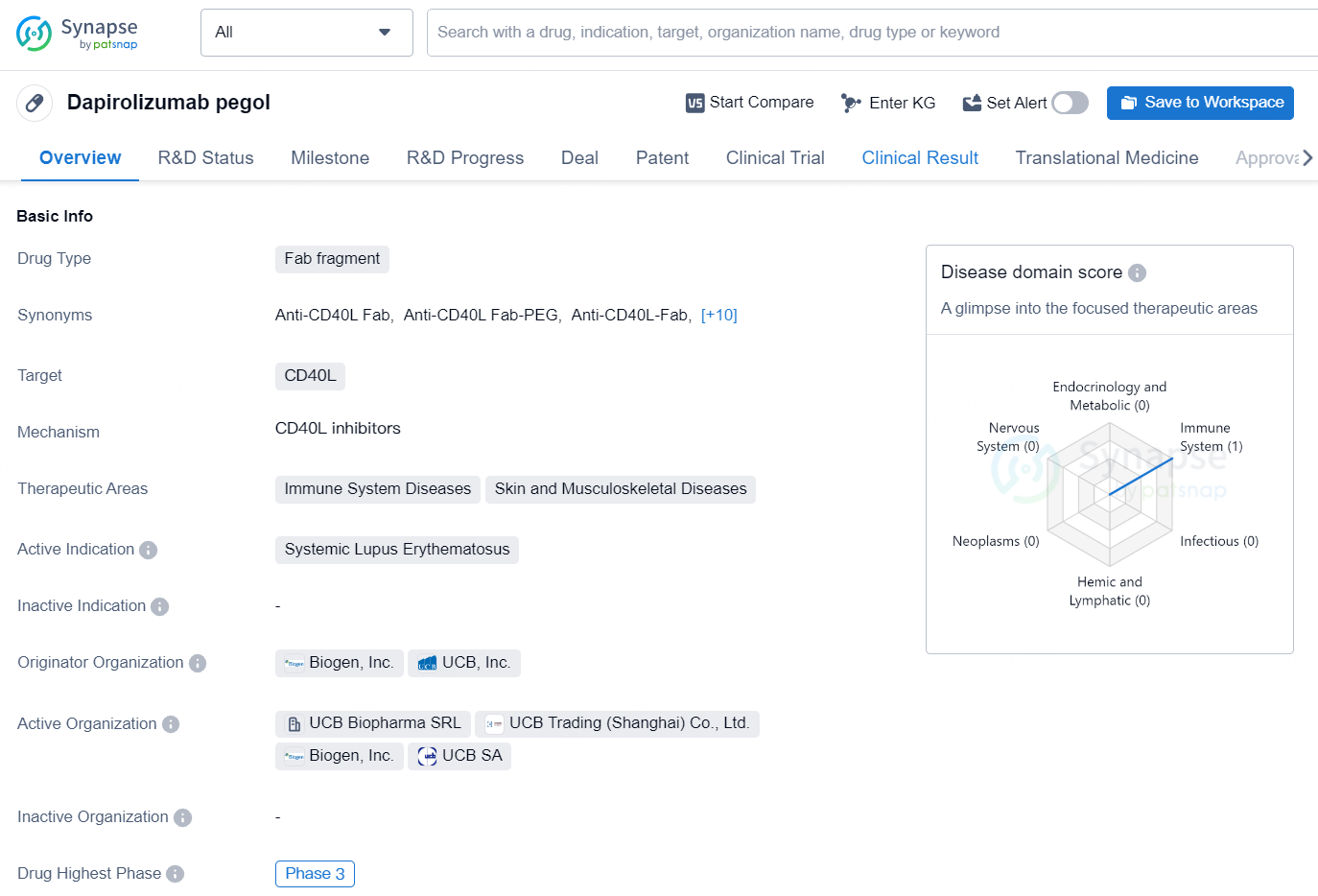
Dapirolizumab pegol is a Fab fragment drug designed to target CD40L and is mainly indicated for systemic lupus erythematosus. It is being developed by Biogen, Inc. and UCB, Inc., and has reached the highest phase of clinical development globally, which is Phase 3. In China, it has also reached the highest phase of development, which is also Phase 3.