Locus Biosciences Secures $23.9M BARDA Investment for Phase 2 of Groundbreaking CRISPR Phage Treatment
Locus Biosciences, Inc., an enterprise specializing in biotechnology at the clinical trial phase, is focused on crafting innovative, precision-based bacteriophage therapies to combat a wide array of bacterial infections. The company has recently declared that it has secured the disbursement of $23.9 million in funds from the Biomedical Advanced Research and Development Authority, which operates under the umbrella of the Administration for Strategic Preparedness and Response in the United States.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Health and Human Services Department will maintain its support for the advancement of Locus' genetic-editing CRISPR technology in LBP-EC01, a phage-based treatment aimed at combatting urinary infections that are non-responsive to traditional drugs and are provoked by Escherichia coli. This decision follows encouraging data from an initial Phase 2a clinical study.
Globally, urinary tract infections (UTIs) impact a staggering 150 million individuals every year. E. coli is the primary culprit in approximately 80% of these cases, with many instances involving antibiotic-resistant variants that defy standard medical interventions. Recurrence rates for UTI can reach 40%, with repeat infections typically occurring a few months after initial treatment.
Both the U.S. Centers for Disease Control and Prevention and the World Health Organization consider antibiotic-resistant E. coli to be a critical and immediate threat to global health, highlighting the urgent need for novel therapeutic options.
Paul Garofolo, the Co-founder and CEO of Locus, remarks, "Modified bacteriophage therapy stands out as one of the leading solutions to the escalating global health crisis posed by bacteria that survive despite multiple drug treatments. While preliminary trials are promising, the sector is in dire need of a large-scale, placebo-controlled study to definitively determine the success of this genetically modified phage therapy."
In the year 2020, a partnership was revealed between Locus and the Biomedical Advanced Research and Development Authority (BARDA) to jointly finance the progression of LBP-EC01. According to the agreement, BARDA will allocate up to $85 million to Locus as a portion of a comprehensive $152 million initiative. This investment will bolster Phase 2 and Phase 3 clinical trials and back additional measures necessary for acquiring the U.S. Food and Drug Administration's marketing authorization for LBP-EC01. The current announcement confirms a total release of $48.9 million from the budget under this agreement.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
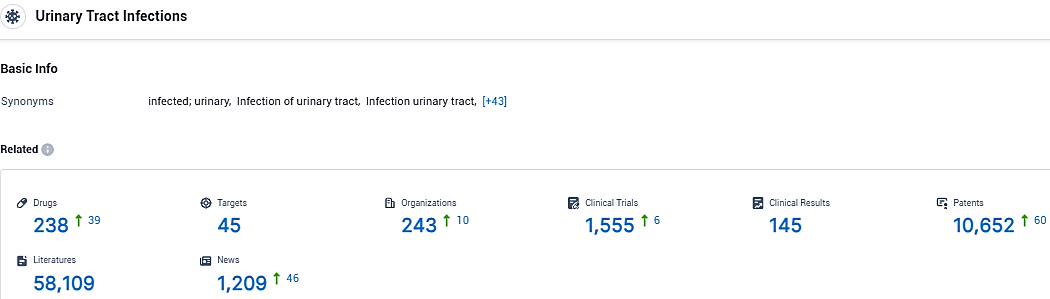
According to the data provided by the Synapse Database, As of January 29, 2024, there are 238 investigational drugs for the urinary tract infections, including 45 targets, 243 R&D institutions involved, with related clinical trials reaching 1555, and as many as 10652 patents.
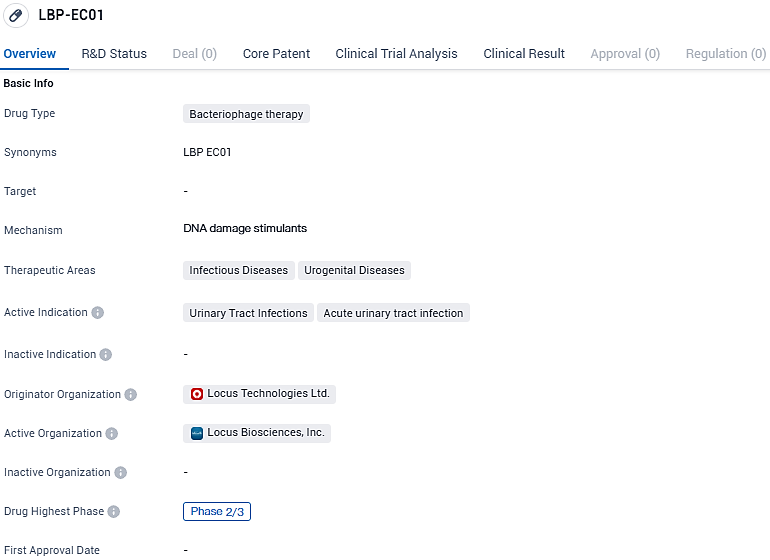
LBP-EC01 is a bacteriophage therapy being developed by Locus Technologies Ltd. for the treatment of urinary tract infections, specifically acute urinary tract infections. This therapy holds promise as an alternative to antibiotics and has reached Phase 2/3 of development, indicating progress in its clinical testing.