MacroGenics Signs Deal with TerSera Therapeutics to Sell MARGENZA®
MacroGenics, Inc. (NASDAQ: MGNX), a company specializing in the discovery, development, production, and commercialization of innovative antibody therapies for cancer treatment, has revealed its agreement with TerSera Therapeutics LLC, a privately-owned biopharmaceutical firm concentrating on oncology and non-opioid pain management. Under this agreement, TerSera will obtain the worldwide rights to MARGENZA® (margetuximab-cmkb).
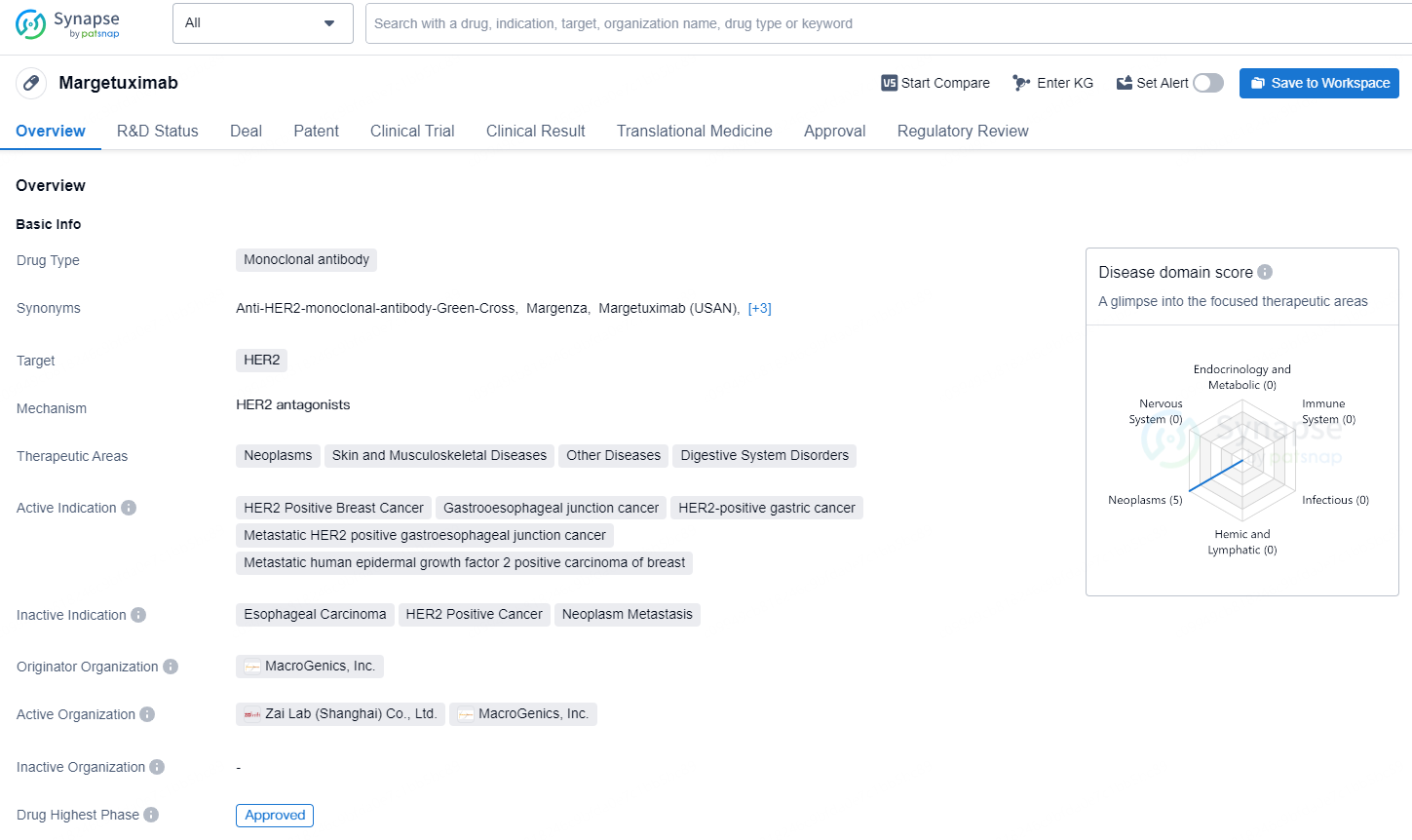
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
MARGENZA received approval from the U.S. Food and Drug Administration (FDA) in December 2020 for use alongside chemotherapy in adult patients with metastatic HER2-positive breast cancer who have undergone two or more prior anti-HER2 treatments, at least one of which must have been for metastatic disease. This approval was founded on findings from the pivotal Phase 3 clinical trial (SOPHIA), which compared the safety and effectiveness of MARGENZA with Herceptin® (trastuzumab), both administered with chemotherapy.
Under the agreement, TerSera will remit $40 million to MacroGenics upon closing. Additionally, MacroGenics may obtain further milestone payments that could total up to $35 million. The transaction is projected to finalize in the fourth quarter of 2024, contingent on standard closing requirements.
“This agreement will allow us to concentrate on progressing our array of innovative and distinct oncology product candidates,” stated Scott Koenig, M.D., Ph.D., President and CEO of MacroGenics. “We believe that TerSera’s established and complementary commercial framework in the U.S. could enhance patient access to MARGENZA.”
“MARGENZA represents a vital treatment alternative for individuals battling metastatic HER2+ breast cancer,” remarked Edward Donovan, CEO of TerSera. “We are thrilled to integrate MARGENZA into our current oncology offerings, reinforcing our commitment to supporting breast cancer patients.”
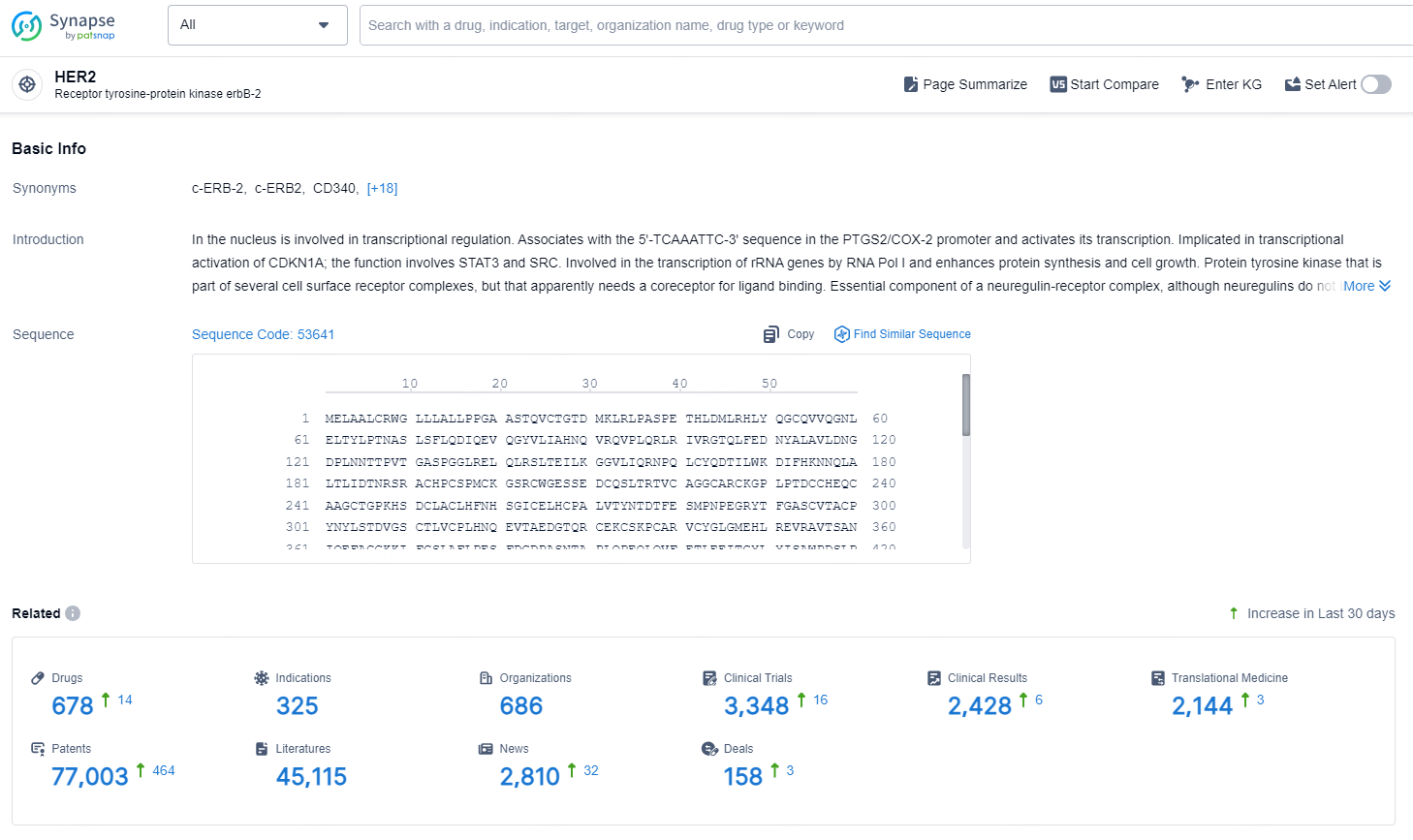
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 678 investigational drugs for the HER2 targets, including 325 indications, 686 R&D institutions involved, with related clinical trials reaching 3348, and as many as 77003 patents.
Margetuximab is a monoclonal antibody drug that targets HER2, a protein that can promote the growth of cancer cells. The therapeutic areas for this drug include neoplasms, skin and musculoskeletal diseases, other diseases, and digestive system disorders. The active indications for Margetuximab are HER2 Positive Breast Cancer, Gastroesophageal junction cancer, HER2-positive gastric cancer, metastatic HER2 positive gastroesophageal junction cancer, and metastatic human epidermal growth factor 2 positive carcinoma of breast.