Zymeworks Initiates Phase 1 Trial of ZW171 in Mesothelin-Expressing Advanced Cancers
Zymeworks Inc. (Nasdaq: ZYME), a biotechnology firm in the clinical phase, is advancing a varied portfolio of innovative, multifunctional biotherapeutics aimed at enhancing treatment standards for hard-to-manage diseases. The company has announced that the initial participant has been administered the investigational therapy ZW171 as part of the first-in-human Phase 1 trial (NCT06523803), which is focused on assessing the safety and tolerability of this treatment for patients with advanced or metastatic ovarian cancer, non-small cell lung cancer (NSCLC), and other malignancies expressing MSLN.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
MSLN exhibits significant expression across various tumor types, notably in ovarian cancer and moderately in NSCLC. This expression profile makes it a promising candidate for therapeutic intervention using our proprietary T cell engager technology. Preclinical investigations have shown that ZW171 effectively induces strong in vivo selective destruction of target cells overexpressing MSLN and enhances MSLN-related T cell activation. This minimizes the likelihood of on-target-off-tumor adverse effects, as well as reduces the risk of peripheral T cell activation and cytokine release syndrome.
Data shared at the 2023 American Association for Cancer Research Annual Meeting indicate that ZW171 displays superior anti-tumor effectiveness relative to benchmarks in MSLN-positive tumor models and is well tolerated in cynomolgus monkeys at doses up to 30 mg/kg2.
“We are excited to have commenced the clinical assessment of ZW171 for patients with ovarian cancer and NSCLC, as it shows the potential to be a highly effective treatment with a favorable safety profile based on our preclinical findings,” stated Jeff Smith, M.D., FRCP, Executive Vice President and Chief Medical Officer at Zymeworks. “Starting this trial represents a noteworthy advancement in our goal to introduce a new therapeutic option for patients with challenging cancers and underscores our ambition to move forward with two therapeutic candidates, ZW171 and ZW191, into clinical trials in 2024.”
The Phase 1 trial consists of two segments, is open-label, and is multi-center, projected to enroll around 160 adult patients suffering from advanced MSLN-overexpressing cancers. The first part of the trial will assess the safety and tolerability of ZW171, including dose escalation among participants with advanced ovarian cancer and NSCLC; secondary outcomes will gauge pharmacokinetics and confirmed objective response rates. The second segment will expand doses into three groups (ovarian cancer, NSCLC, and a mixed cohort for any MSLN-positive individuals) and will investigate the anti-tumor efficacy of ZW171, with primary focus on safety and tolerability and secondary endpoints evaluating progression-free survival, duration of response, and overall survival. The Company plans to execute the Phase 1 study at research sites across the United States, Europe, and the Asia-Pacific region.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
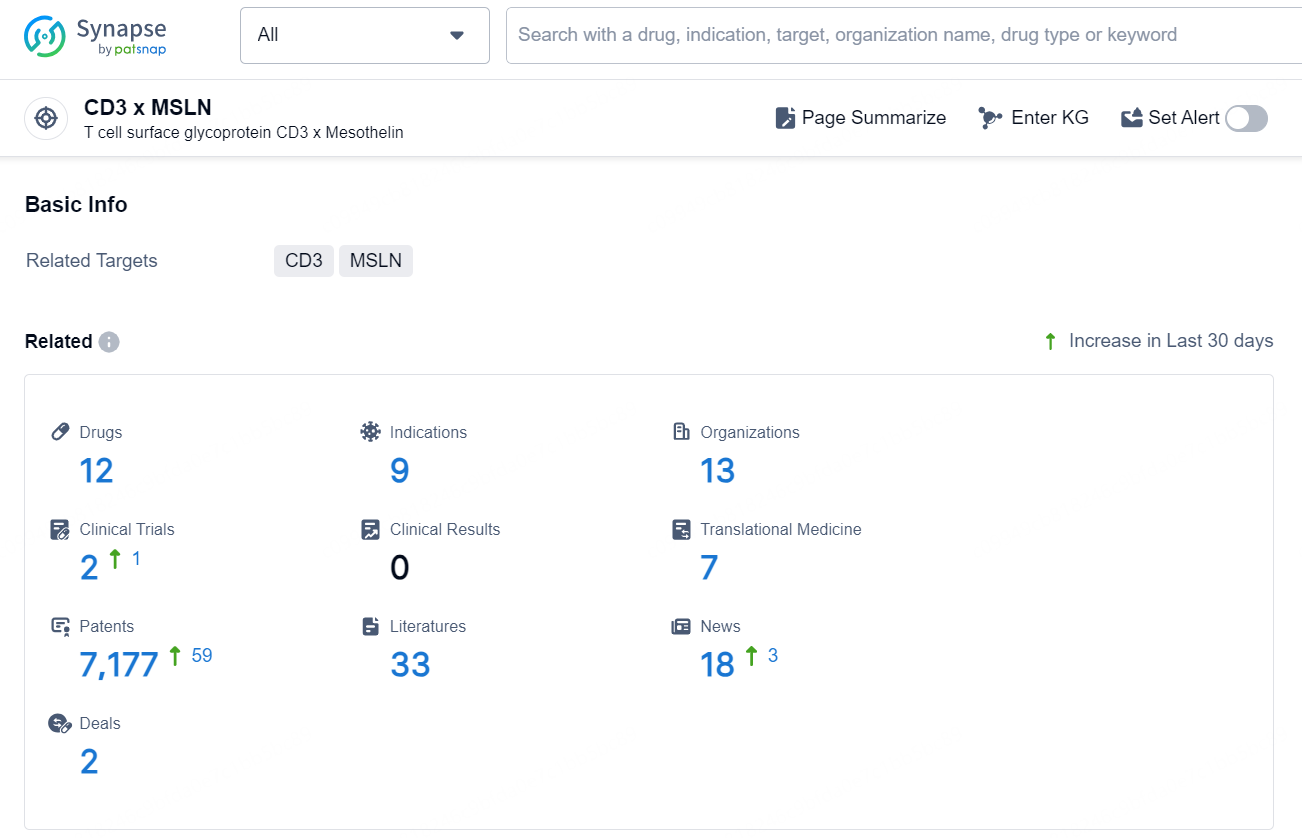
According to the data provided by the Synapse Database, As of October 21, 2024, there are 12 investigational drug for the CD3 x MSLN target, including 9 indications, 13 R&D institutions involved, with related clinical trials reaching 2, and as many as 7177 patents.
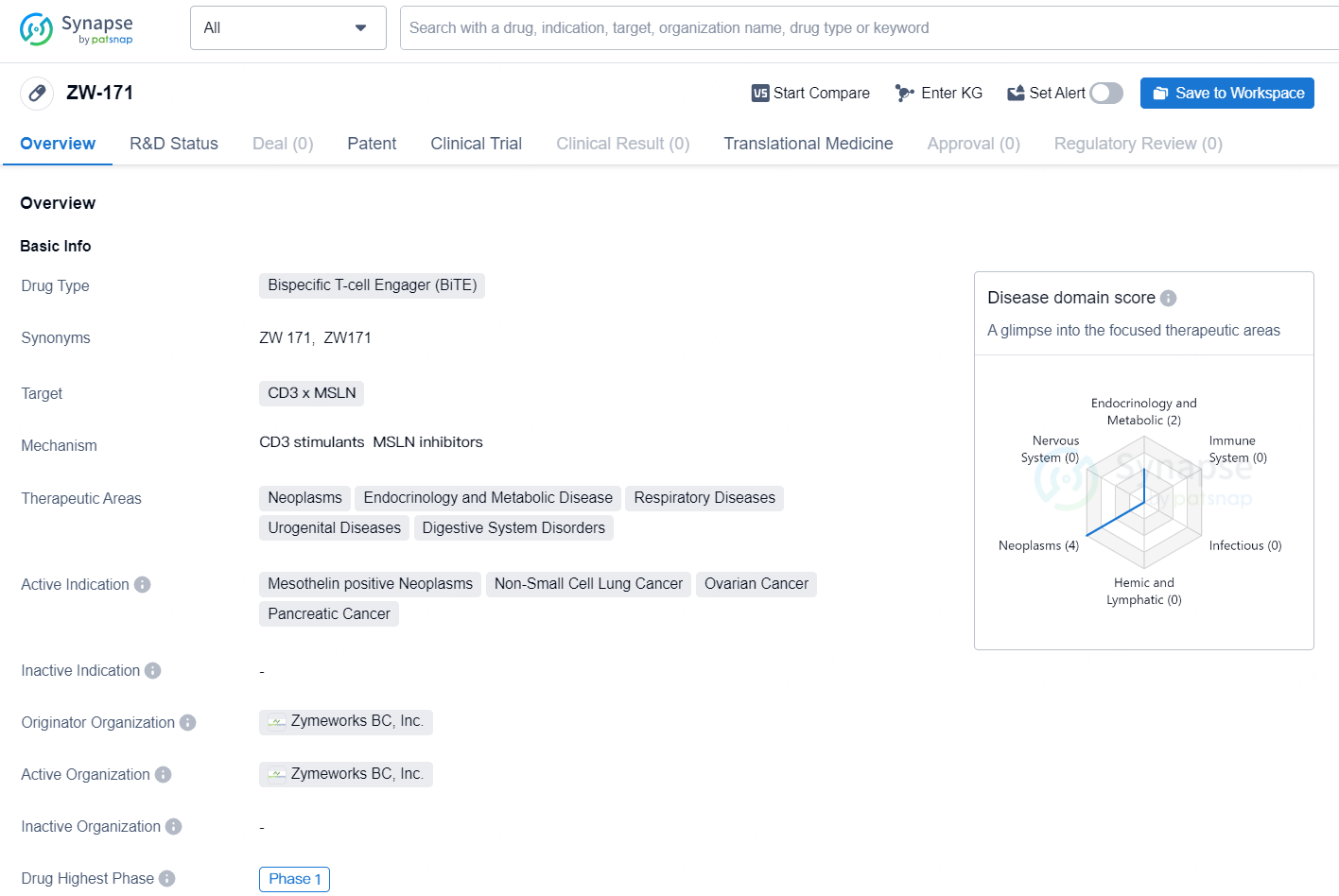
The drug ZW-171 is a Bispecific T-cell Engager (BiTE) that targets CD3 x MSLN. It falls under the therapeutic areas of neoplasms, endocrinology and metabolic disease, respiratory diseases, urogenital diseases, and digestive system disorders. The active indications for ZW-171 include mesothelin positive neoplasms, non-small cell lung cancer, ovarian cancer, and pancreatic cancer.