Nurix Therapeutics Reports Promising Results for BTK Degrader NX-5948 in Waldenström’s Macroglobulinemia Study
Nurix Therapeutics, Inc. (Nasdaq: NRIX), a biopharmaceutical company at the clinical stage that focuses on developing drugs for targeted protein modulation aimed at treating cancer and inflammatory conditions, announced the presentation of clinical findings from its ongoing Phase 1a/1b trial of NX-5948. This compound is an orally bioavailable, brain-penetrant degrader of Burton’s tyrosine kinase (BTK) intended for patients with relapsed/refractory Waldenstrom’s macroglobulinemia (WM). This presentation will take place at the 12th International Workshop on Waldenstrom’s Macroglobulinemia (IWWM-12), scheduled to be held in Prague, Czech Republic, from October 17 to 19, 2024.
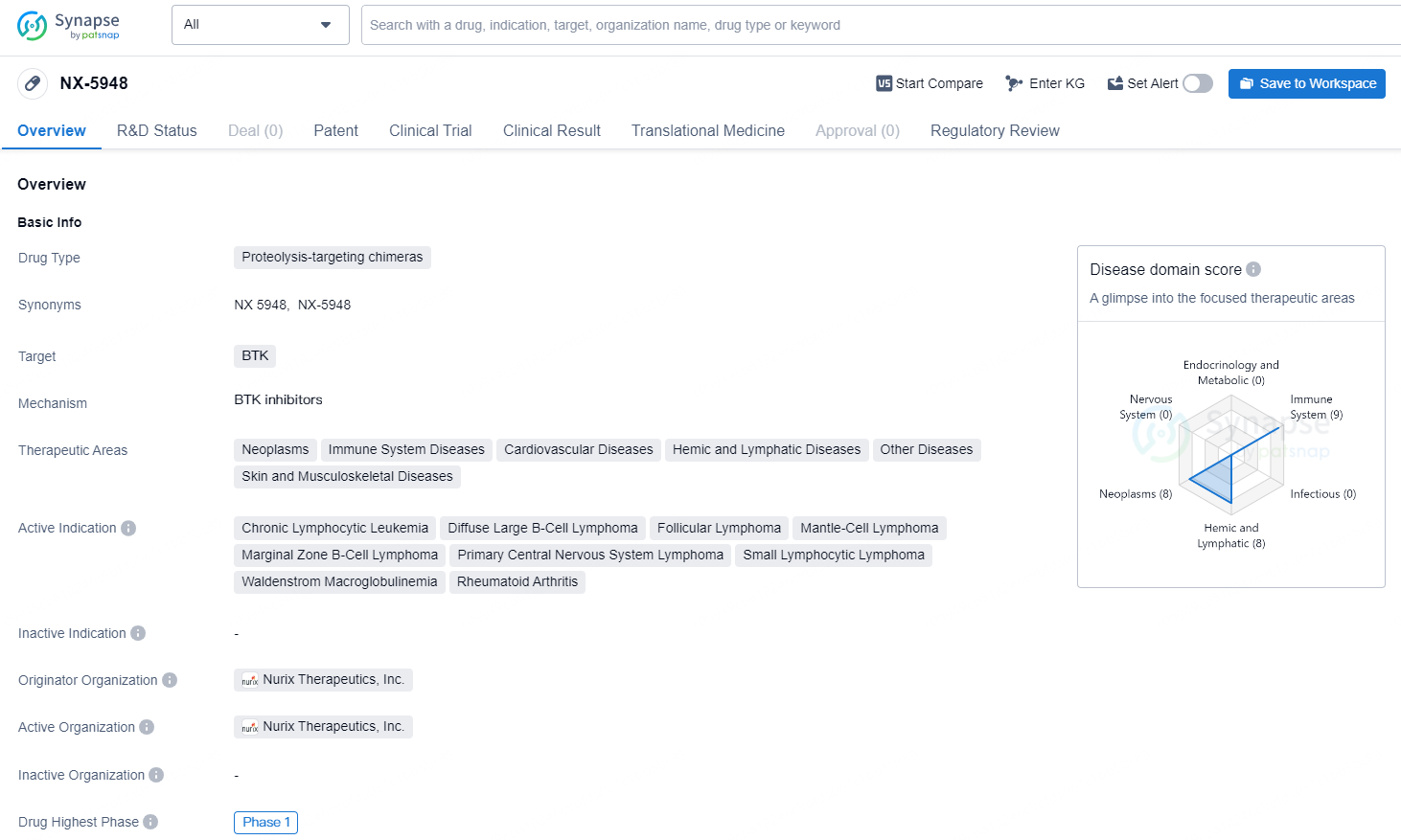
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are heartened by the encouraging emerging data on NX-5948 in individuals with Waldenstrom’s macroglobulinemia, which complements the previously reported significant clinical efficacy noted in chronic lymphocytic leukemia patients," stated Paula G. O’Connor, M.D., the chief medical officer of Nurix. "These findings reinforce our choice to progress NX-5948 into the ongoing Phase 1b expansion cohort involving patients who have undergone at least one prior line of therapy, including a BTK inhibitor, as well as those with Bing-Neel syndrome, a rare variant of WM that affects the central nervous system, where NX-5948’s capacity to cross the blood-brain barrier may provide a distinct advantage."
The information presented at IWWM-12 included safety data previously reported for all participants in the Phase 1a dose escalation study who received NX-5948 at doses between 50 mg and 600 mg once daily through oral administration, irrespective of diagnosis (n=79), as of the data cut on April 17, 2024. NX-5948 exhibited a manageable safety profile, and the safety data for the WM patient cohort aligned with the safety profile seen in the overall group (specific safety data for WM patients was not presented separately).
New information from a data cut on October 10, 2024, details the baseline characteristics of the initial 13 WM patients who were enrolled in both the Phase 1a and Phase 1b segments of the trial, clinical response evaluations in 9 patients who were assessable for response, and the duration of treatment for all 13 patients. The median age of the 13 WM patients was 74 years, and they had a median of 3 prior treatment lines. All patients had previously been treated with both BTK inhibitors (BTKi) and chemotherapy/chemo-immunotherapy.
Among these patients, three (23.1%) had prior exposure to the non-covalent BTKi pirtobrutinib, while one patient (7.7%) had received treatment with a BCL2 inhibitor. Baseline mutation data for MYD88 and CXCR4 were gathered from patient records, revealing that eight patients (61.5%) had MYD88 mutations, and two patients (15.4%) had CXCR4 mutations. In the group of nine patients evaluable for response, seven (77.8%) achieved an objective response, and two (22.2%) exhibited stable disease. All seven responses were noted at the first evaluation conducted at 8 weeks, with five patients continuing treatment and two persisting for over one year. Responses were seen in patients regardless of their MYD88 and CXCR4 mutation statuses.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 21, 2024, there are 307 investigational drugs for the BTK target, including 222 indications, 249 R&D institutions involved, with related clinical trials reaching 1338, and as many as 19313 patents.
The drug NX-5948 is classified as a proteolysis-targeting chimera and targets BTK. Its therapeutic areas include neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The active indications for NX-5948 include chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, mantle-cell lymphoma, marginal zone B-cell lymphoma, primary central nervous system lymphoma, small lymphocytic lymphoma, Waldenstrom macroglobulinemia, and rheumatoid arthritis. The drug originates from Nurix Therapeutics, Inc., and it has reached the highest phase of development at the global level, which is Phase 1.