Otsuka Releases Positive Phase 3 Results for Sibeprenlimab in Adult IgA Nephropathy
Pharmaceutical Development & Commercialization, Inc. and Otsuka Pharmaceutical Co., Ltd. (Otsuka) have announced encouraging topline interim results from the ongoing Phase 3 clinical study of sibeprenlimab aimed at treating immunoglobulin A nephropathy (IgA nephropathy) in adult patients.
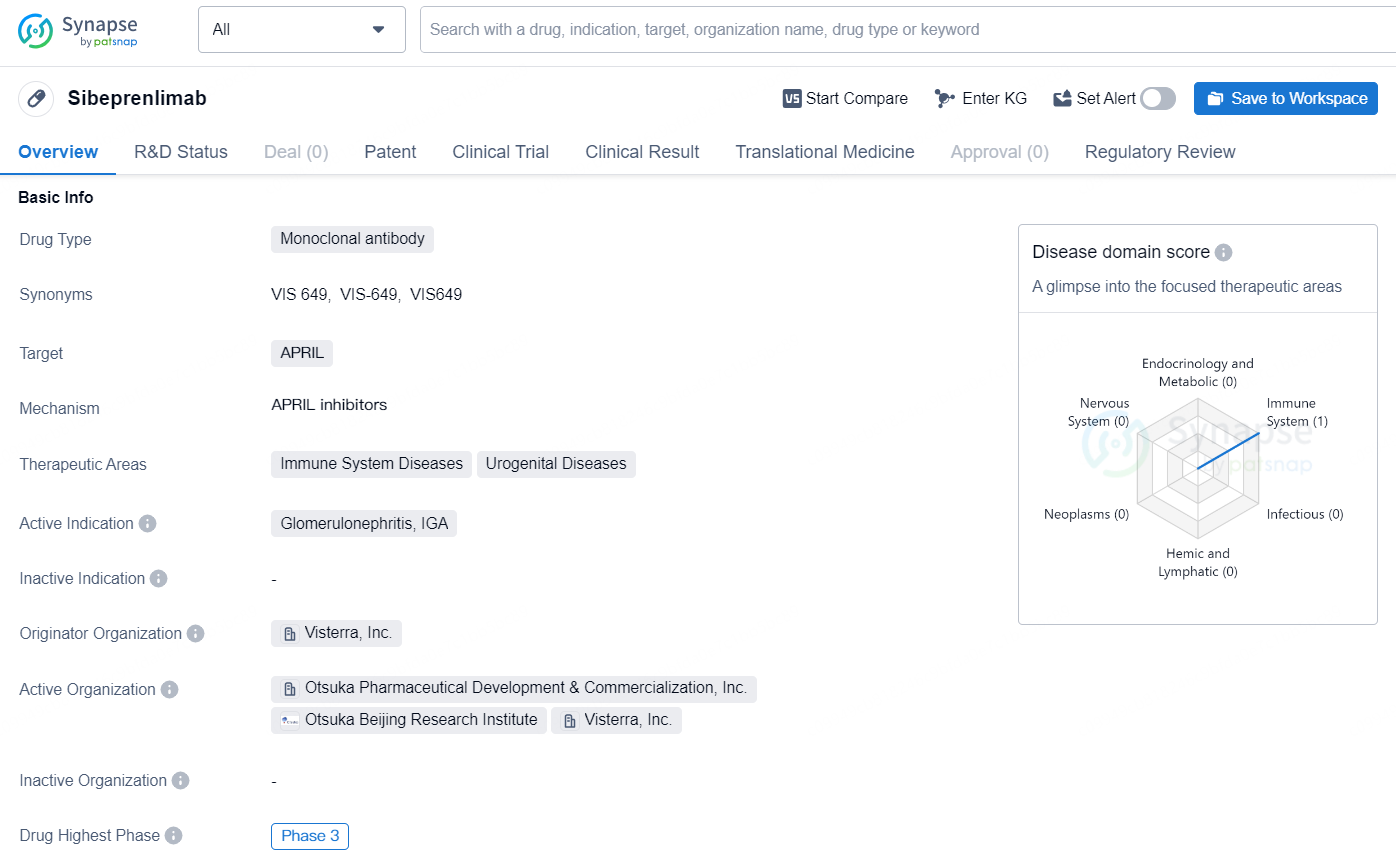
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Sibeprenlimab is an experimental anti-APRIL monoclonal antibody (A PRoliferation-Inducing Ligand) that inhibits a crucial initial phase in the immune pathogenic mechanism of IgA nephropathy by reducing Gd-IgA1 synthesis and the formation of immune complexes. This condition is a chronic autoimmune kidney disease that can progressively result in end-stage kidney disease (ESKD) for many affected individuals. Otsuka received Breakthrough Therapy designation for sibeprenlimab after promising outcomes were reported in the Phase 2 ENVISION clinical trial.
An independent data monitoring committee conducted a pre-defined interim analysis and confirmed that the Phase 3 VISIONARY study (NCT05248646) fulfilled its primary objective, showing that sibeprenlimab led to a statistically significant and clinically relevant decrease in 24-hour uPCR (urine protein-to-creatinine ratio) compared to a placebo group following nine months of treatment. This multicenter, randomized, double-blind, placebo-controlled study involved around 530 adult patients with IgA nephropathy, all receiving standard-of-care therapies (maximally tolerated doses of ACE inhibitors or ARBs +/- SGLT2 inhibitors), and aimed to assess the efficacy and safety of 400 mg of sibeprenlimab given subcutaneously every four weeks against a placebo. The primary efficacy measure was the change in 24-hour uPCR at the 9-month mark, relative to baseline levels.
John Kraus, M.D., Ph.D., executive vice president and chief medical officer of Otsuka Pharmaceutical Development & Commercialization, Inc., commented, “The encouraging interim results from this study imply that by targeting APRIL, we may establish a novel therapeutic approach for those affected by this advancing kidney condition. We eagerly anticipate concluding this study and discussing the complete findings at a later date, with deep gratitude to the IgA nephropathy patients, their caregivers, and the investigators who have greatly contributed to this research.”
Brian Pereira, M.D., CEO of Visterra, Inc., an affiliate of Otsuka in the U.S. responsible for the design and development of sibeprenlimab, expressed, “We are heartened by the ongoing advancements of sibeprenlimab and its potential to offer a significant and possibly disease-modifying treatment alternative for patients with IgA nephropathy.”
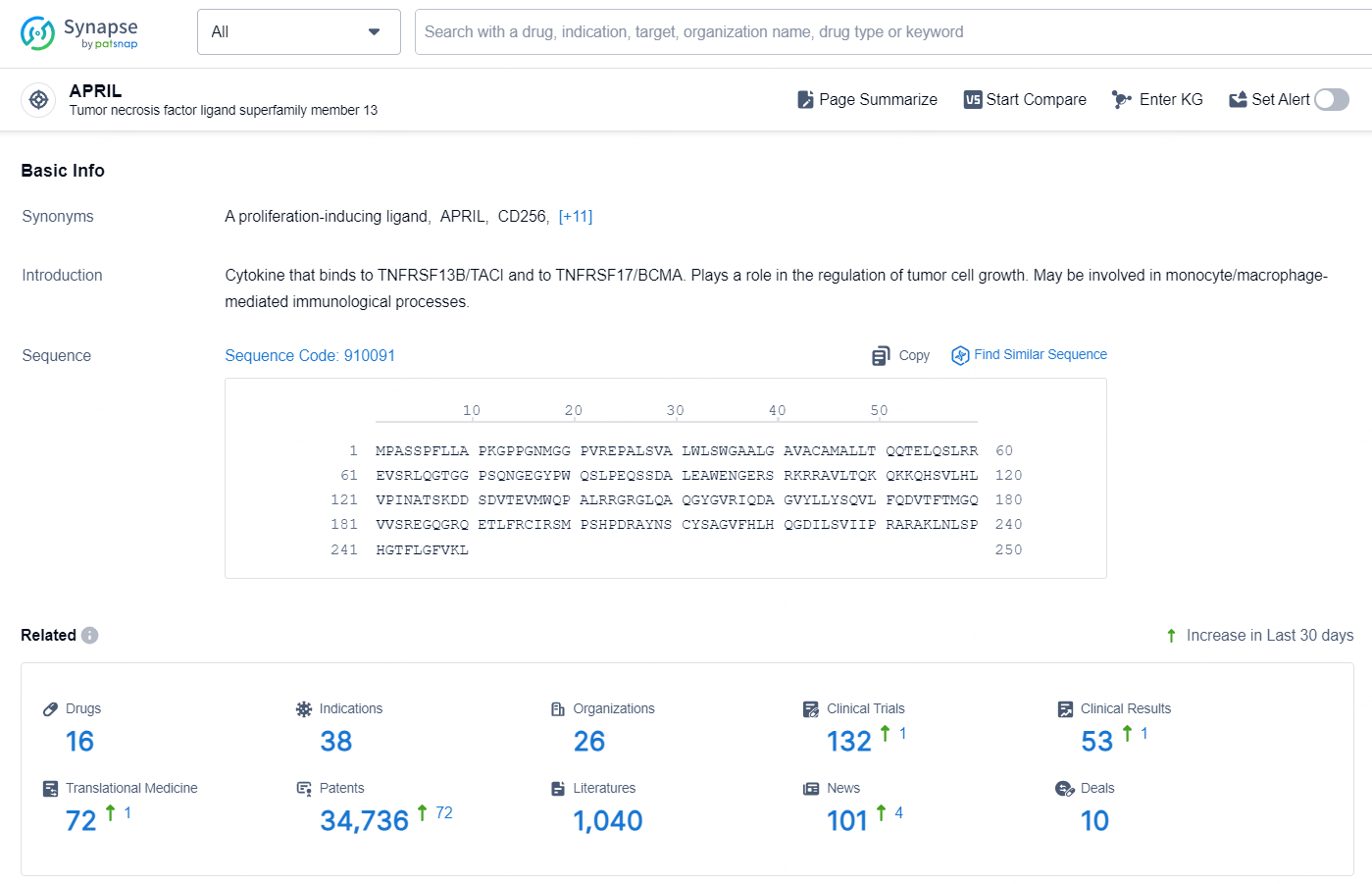
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 16 investigational drug for the APRIL targets, including 38 indications, 26 R&D institutions involved, with related clinical trials reaching 132, and as many as 34736 patents.
Sibeprenlimab is a monoclonal antibody drug that targets APRIL and is primarily intended for the treatment of immune system and urogenital diseases. The active indication for Sibeprenlimab is Glomerulonephritis, IGA. The drug is developed by Visterra, Inc., and has reached the highest Phase of clinical development, which is Phase 3, both globally and in China.