Merck KGaA Begins Phase III Trial of Oral Cladribine for gMG in Germany
Merck KGaA, Darmstadt, Germany, a prominent science and technology enterprise, disclosed the commencement of dosing in the Phase III MyClad clinical trial (NCT06463587), which assesses the therapeutic efficacy and safety profile of oral cladribine in the management of generalized Myasthenia Gravis (gMG). The cladribine capsules hold promise as a pioneering oral therapeutic option for gMG patients. Generalized Myasthenia Gravis (gMG) represents a rare, neuromuscular ailment characterized by muscular weakness, which can escalate into severe conditions significantly altering patients' quality of life.
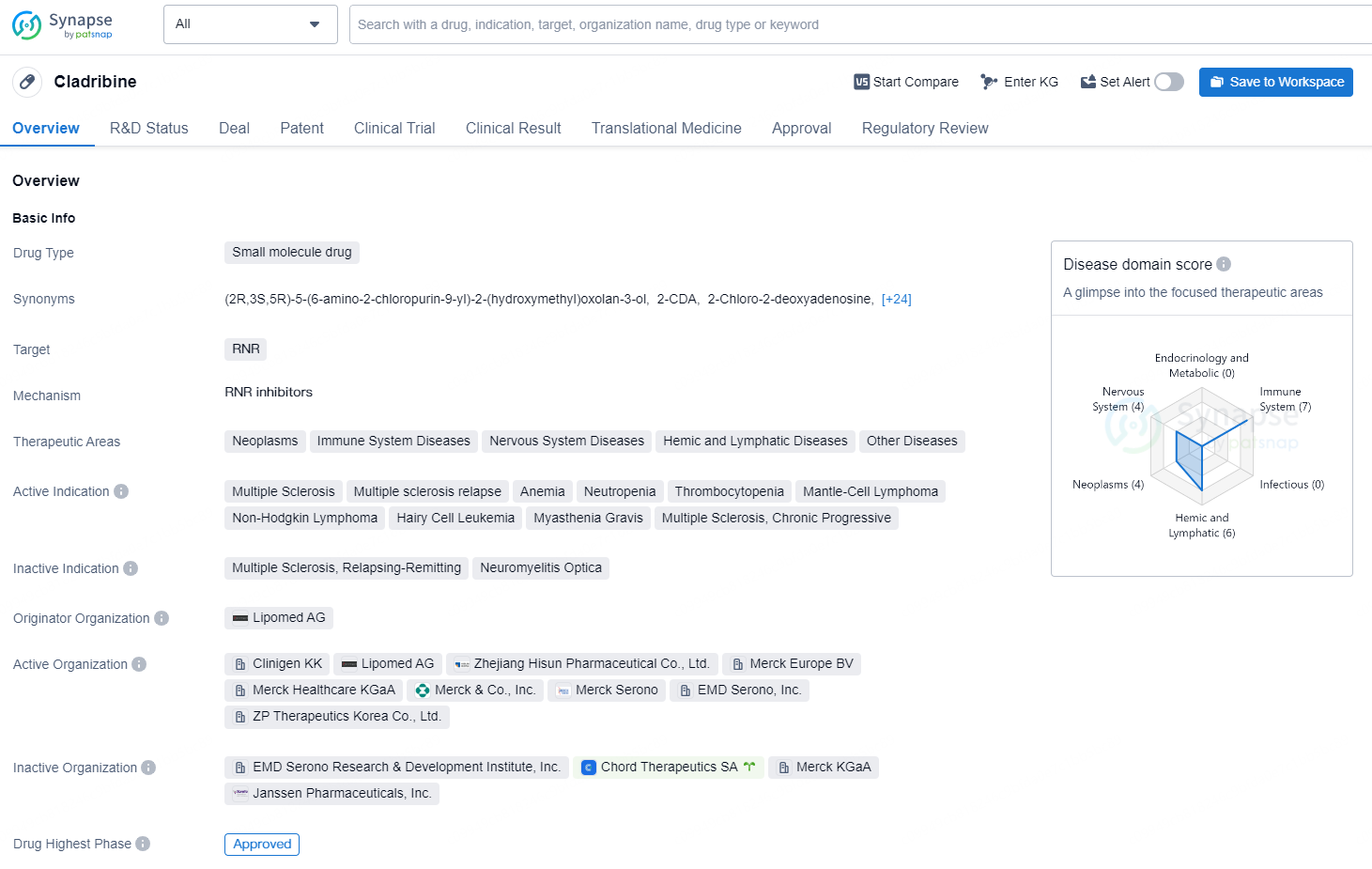
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Cladribine is anticipated to precisely target B and T lymphocytes, which are hypothesized to be the underlying contributors to gMG by producing detrimental autoantibodies that ignite inflammation at the nerve-muscle interfaces. This unique mechanism of action, paired with a concise, home-administered oral dosing regimen, has the potential to mitigate disease progression by addressing its fundamental cause while minimizing the therapeutic burden.
"Drawing from our vast expertise in catering to the needs of patients with immune-mediated neurological conditions, we are confident that cladribine capsules represent a distinctively promising therapeutic avenue for gMG," remarked Jan Klatt, Head of Development Unit Neurology & Immunology, Merck KGaA, Darmstadt, Germany, Healthcare Division. "This therapeutic strategy aims to attain a superior level of disease activity management, significantly enhancing convenience and ultimately empowering patients to lead lives as close to normalcy as possible."
The MyClad trial is a global Phase III, randomized, double-blind, placebo-controlled study, designed to rigorously evaluate the effectiveness and safety of cladribine capsules in a cohort of 240 gMG patients.
Generalized Myasthenia Gravis (gMG), a rare, chronic autoimmune neuromuscular condition, is marked by muscle weakness and exhaustion, affecting approximately 700,000 individuals globally. While it can affect people of all ages, it is more prevalent among young women (aged 20-30) and men over 50. In gMG, the communication between nerves and muscles, particularly at the neuromuscular junction (NMJ), is disrupted, leading to muscle weakness. This can manifest as loss of eye muscle control and varying degrees of weakness in the arms, legs, and respiratory muscles. The unpredictable severity and frequency of symptoms in gMG patients can be incapacitating, profoundly disrupting various aspects of daily life.
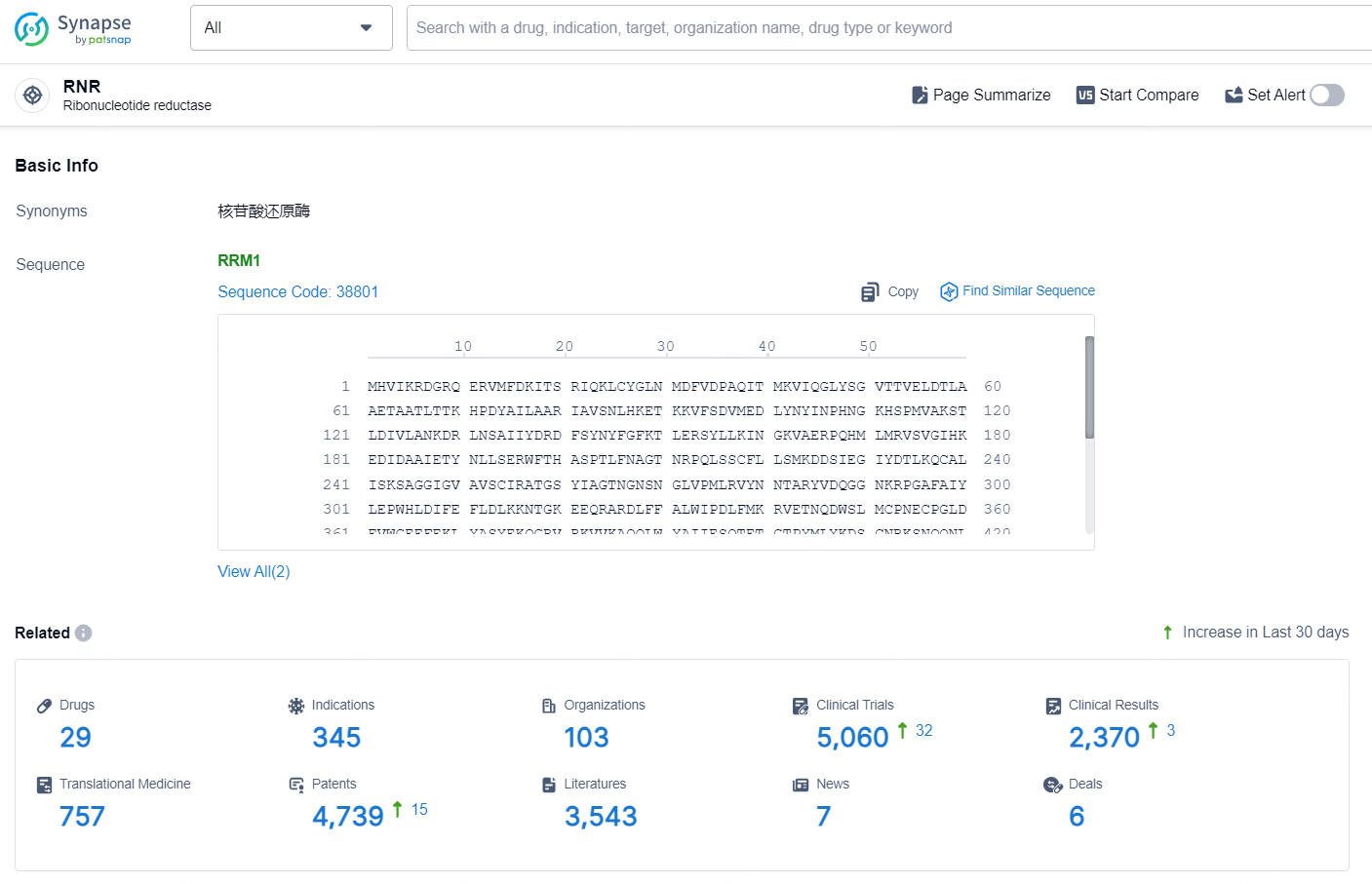
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 2, 2024, there are 29 investigational drugs for the RNR targets, including 345 indications, 103 R&D institutions involved, with related clinical trials reaching 5060, and as many as 4739 patents.
Cladribine is a small molecule drug that targets the enzyme RNR (ribonucleotide reductase) and is used in the treatment of various therapeutic areas including Neoplasms, Immune System Diseases, Nervous System Diseases, Hemic and Lymphatic Diseases, and Other Diseases. The drug is indicated for the treatment of multiple sclerosis, multiple sclerosis relapse, anemia, neutropenia, thrombocytopenia, mantle-cell lymphoma, non-Hodgkin lymphoma, hairy cell leukemia, myasthenia gravis, and chronic progressive multiple sclerosis.