Biocon Biologics Secures Market Access for Biosimilar Bmab 1200 in Europe, UK, Canada, and Japan
Biocon Biologics Ltd (BBL), a comprehensively integrated worldwide player in biosimilars and an affiliate of Biocon Limited (BSE ticker: 532523, NSE: BIOCON), disclosed that it has executed a settlement and licensing accord with Janssen Biotech Inc., Janssen Sciences Ireland, and Johnson & Johnson (jointly referred to as Janssen), which paves the path for the commercial rollout of Bmab 1200, its prospective biosimilar counterpart to Stelara®, in Europe, the United Kingdom (UK), Canada, and Japan.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Pursuant to the settlement accord's conditions, Biocon Biologics has amicably resolved patent disagreements with Janssen, securing market access timelines in Europe, the United Kingdom, Canada, and Japan. Currently, the requisite regulatory submissions in these regions are undergoing scrutiny.
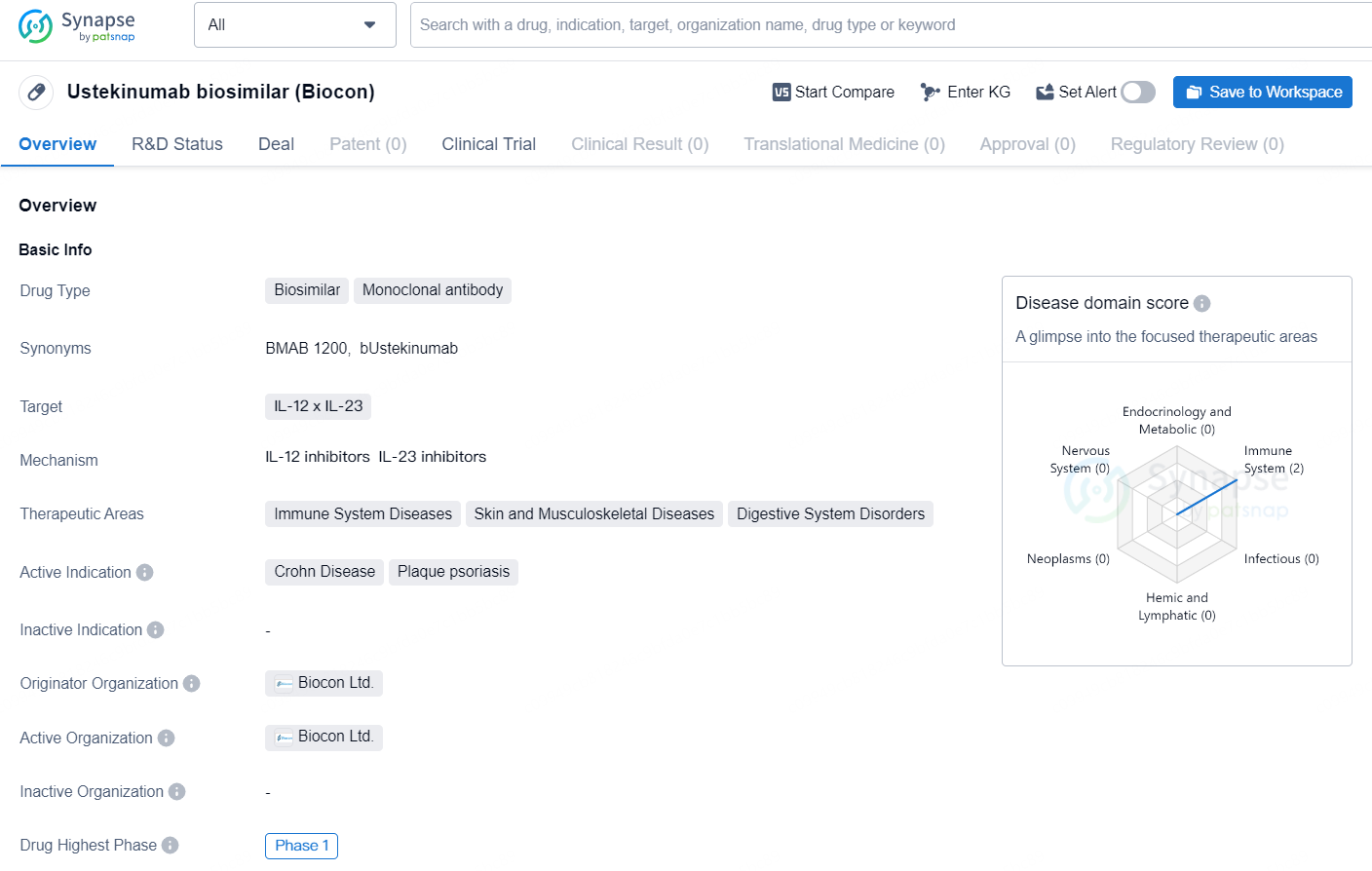
Earlier, Biocon Biologics disclosed a settlement deal in the United States, paving the way for the introduction of Bmab 1200 by February 22, 2025, contingent upon approval from the U.S. FDA. Notably, the FDA has officially accepted the Company's Biologics License Application (BLA) for Bmab 1200 (bUstekinumab) for assessment under the 351(k) regulatory framework.
Shreehas Tambe, CEO and Managing Director of Biocon Biologics Ltd, remarked, "This settlement underscores our robust reputation for scientific excellence and innovation, marking a pivotal achievement in our endeavor to bring our biosimilar Bmab 1200 (bUstekinumab) to global audiences. Bmab 1200 will significantly bolster our immunology portfolio, offering patients suffering from autoimmune conditions a cost-effective and efficacious treatment alternative."
Stelara® (Ustekinumab), a monoclonal antibody therapeutic, works by inhibiting the abnormal regulation of interleukin IL-12/23-mediated immune disorders and has garnered approval for managing psoriasis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis. The reference product, Stelara®, recorded global sales of $10.85 billion in 2023.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
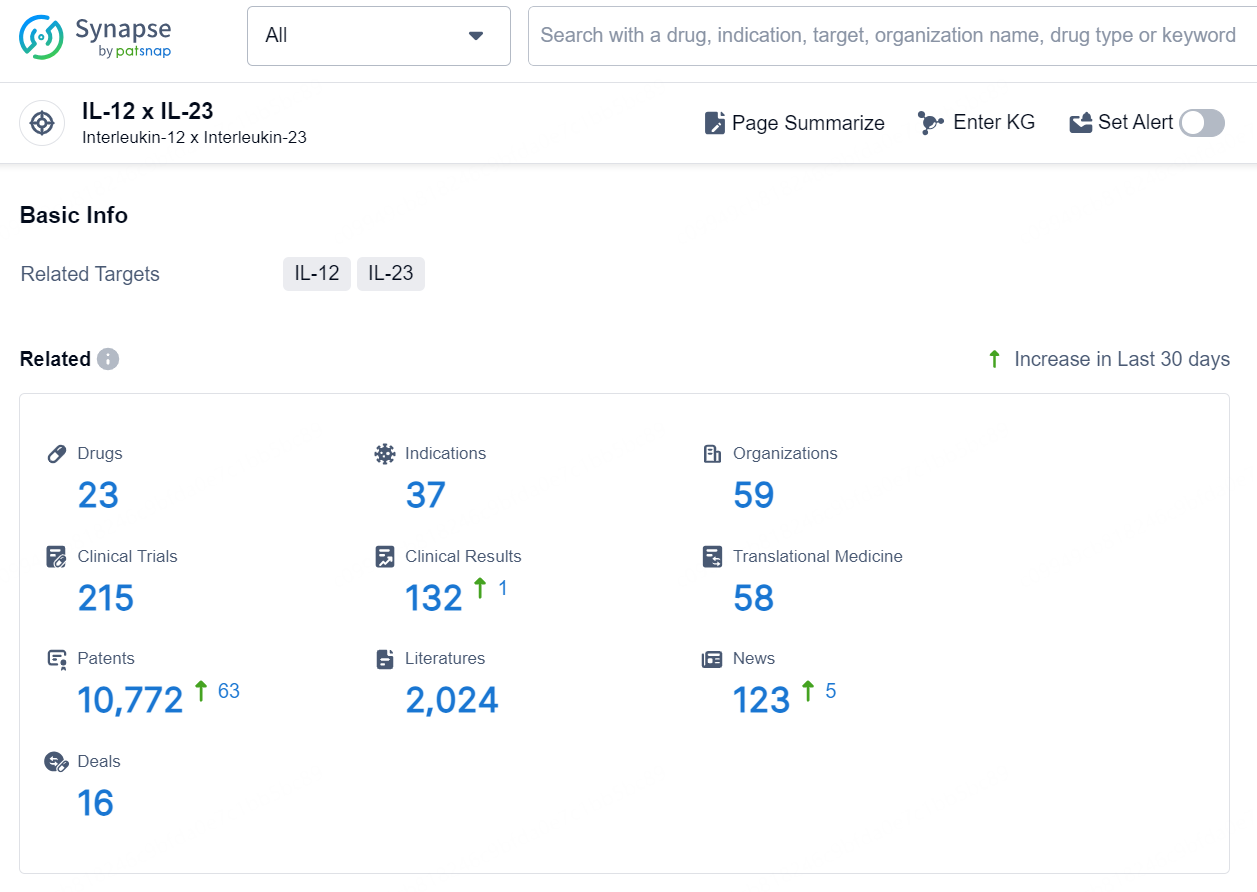
According to the data provided by the Synapse Database, As of September 2, 2024, there are 23 investigational drugs for the IL-12 x IL-23 targets, including 37 indications, 59 R&D institutions involved, with related clinical trials reaching 215, and as many as 10772 patents.
The drug Ustekinumab biosimilar (Biocon) is a biosimilar monoclonal antibody that targets IL-12 and IL-23. It is primarily used for the treatment of immune system diseases, skin and musculoskeletal diseases, and digestive system disorders. The active indications for this drug are Crohn Disease and plaque psoriasis. The drug is developed by Biocon Ltd., and as of the latest information available, it has reached the highest phase of development at Phase 1 globally.