Merck to Present New Data on Anti-TL1A Monoclonal Antibody Tulisokibart (MK-7240) in IBD at UEG Week 2024
Merck (NYSE: MRK), referred to as MSD outside the U.S. and Canada, disclosed that new findings on the long-term effectiveness and safety of tulisokibart (MK-7240), an experimental humanized monoclonal antibody targeting the novel protein tumor necrosis factor (TNF)-like cytokine 1A (TL1A), in treating ulcerative colitis (UC) and Crohn’s disease (CD) will be unveiled at the United European Gastroenterology (UEG) Week 2024 Congress in Vienna, Austria.
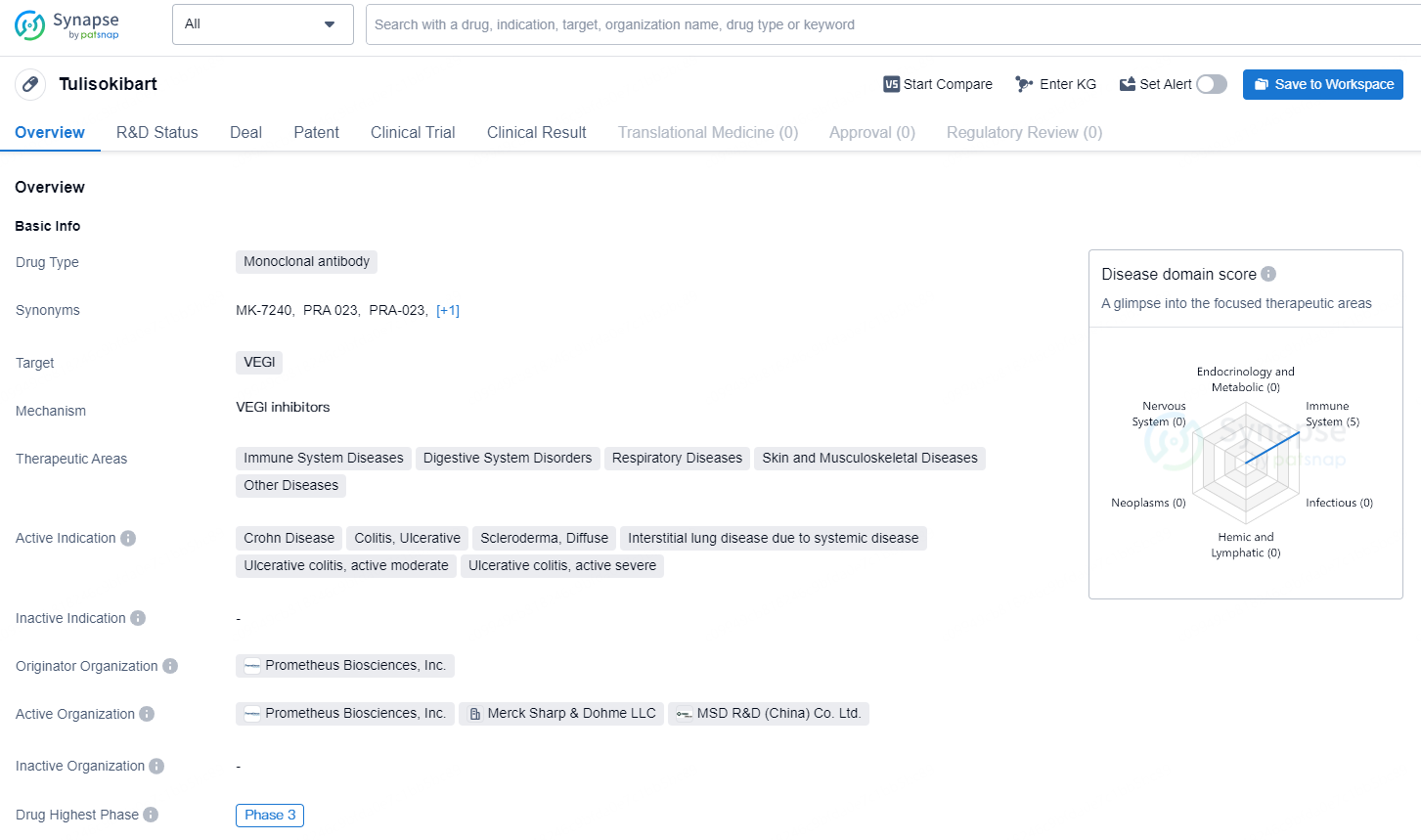
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Long-term efficacy and safety data for tulisokibart from the open-label extension phase of the Phase 2 ARTEMIS-UC and APOLLO-CD studies will be highlighted in two oral presentations. By week 50, both studies showed that those who responded to the 12-week induction phase generally maintained treatment efficacy. The safety profile observed during the study extensions remained consistent with the data previously reported. Key presentation details are as follows:
OP078: Long-term efficacy and safety of tulisokibart in Crohn’s disease (CD): Findings from the open-label extension phase of the Phase 2 APOLLO-CD study; October 14, 10:12-10:24 a.m. CEST
OP194: Long-term efficacy and safety of tulisokibart in ulcerative colitis (UC): Findings from the open-label extension phase of the Phase 2 ARTEMIS-UC study; October 15, 11:54-12:06 p.m. CEST
"We are encouraged by the new maintenance data for tulisokibart in both ulcerative colitis and Crohn’s disease, demonstrating the potential for this novel therapy to help patients achieve long-term clinical remission," stated Dr. Aileen Pangan, vice president, global clinical development, Merck Research Laboratories. "Despite available treatments, many patients with inflammatory bowel disease fail to reach their treatment goals. There remains a need for additional treatment options to help patients manage the tough symptoms of ulcerative colitis and Crohn’s disease."
Merck has launched two Phase 3 studies to investigate the efficacy and safety of tulisokibart in UC (ATLAS-UC; NCT06052059) and CD (ARES-CD; NCT06430801). These represent the first Phase 3 clinical trials for an anti-TL1A antibody in inflammatory bowel disease.
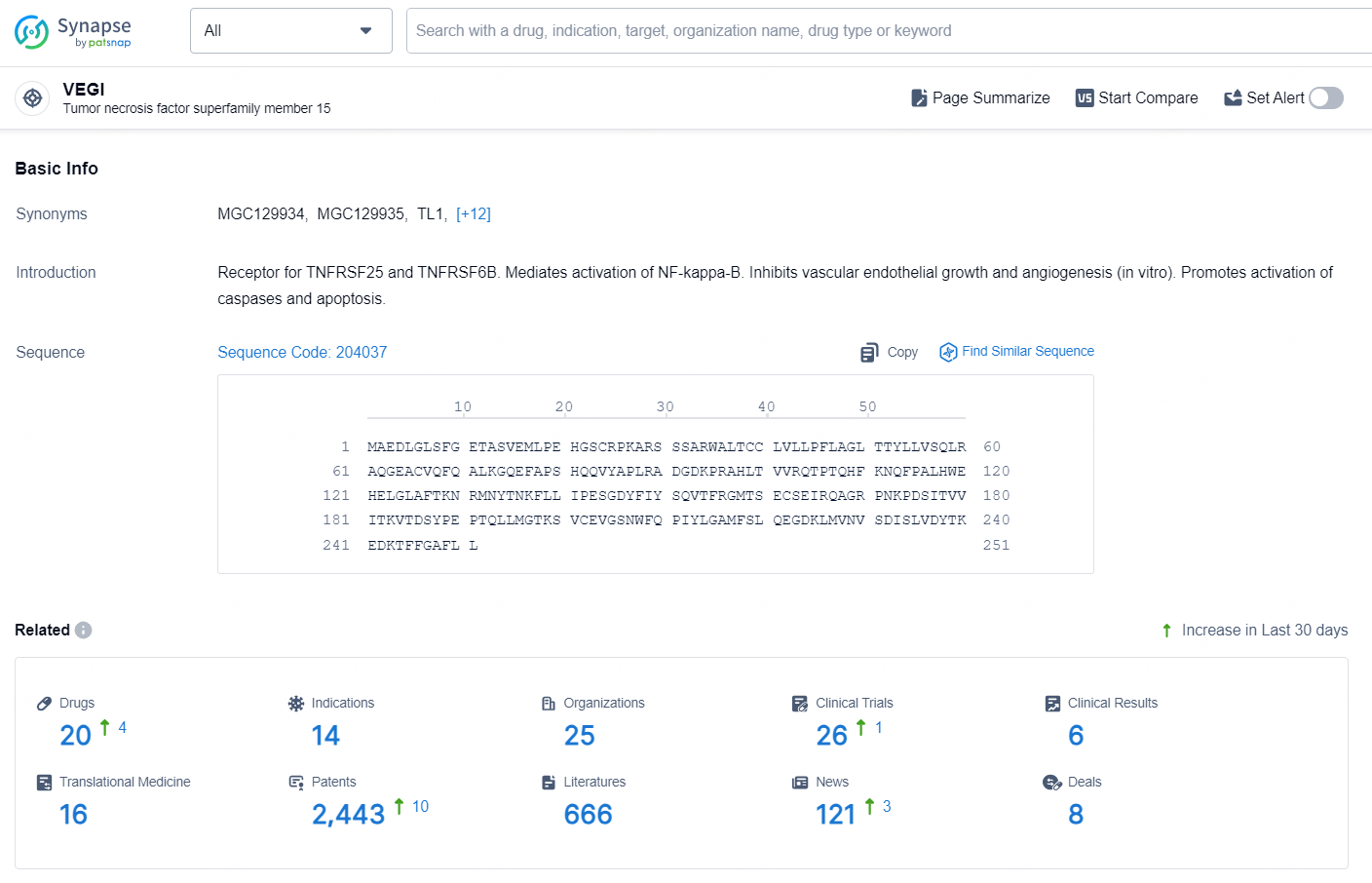
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 30, 2024, there are 46 investigational drug for the VEGI targets, including 104 indications, 75 R&D institution involved, with related clinical trials reaching 1066, and as many as 2361 patents.
Tulisokibart is a monoclonal antibody drug targeting VEGI, which has therapeutic areas in immune system diseases, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, as well as other diseases. The active indications for [Tulisokibart] include Crohn Disease, Colitis, Ulcerative, Scleroderma, Diffuse, Interstitial lung disease due to systemic disease, Ulcerative colitis (active moderate), and Ulcerative colitis (active severe).