Merck's Phase 3 Trial Update on Vibostolimab & Pembrolizumab for Small Cell Lung Cancer
Merck, recognized as MSD outside of the U.S. and Canada, revealed the termination of the Phase 3 KeyVibe-008 trial following advice from an independent Data Monitoring Committee.
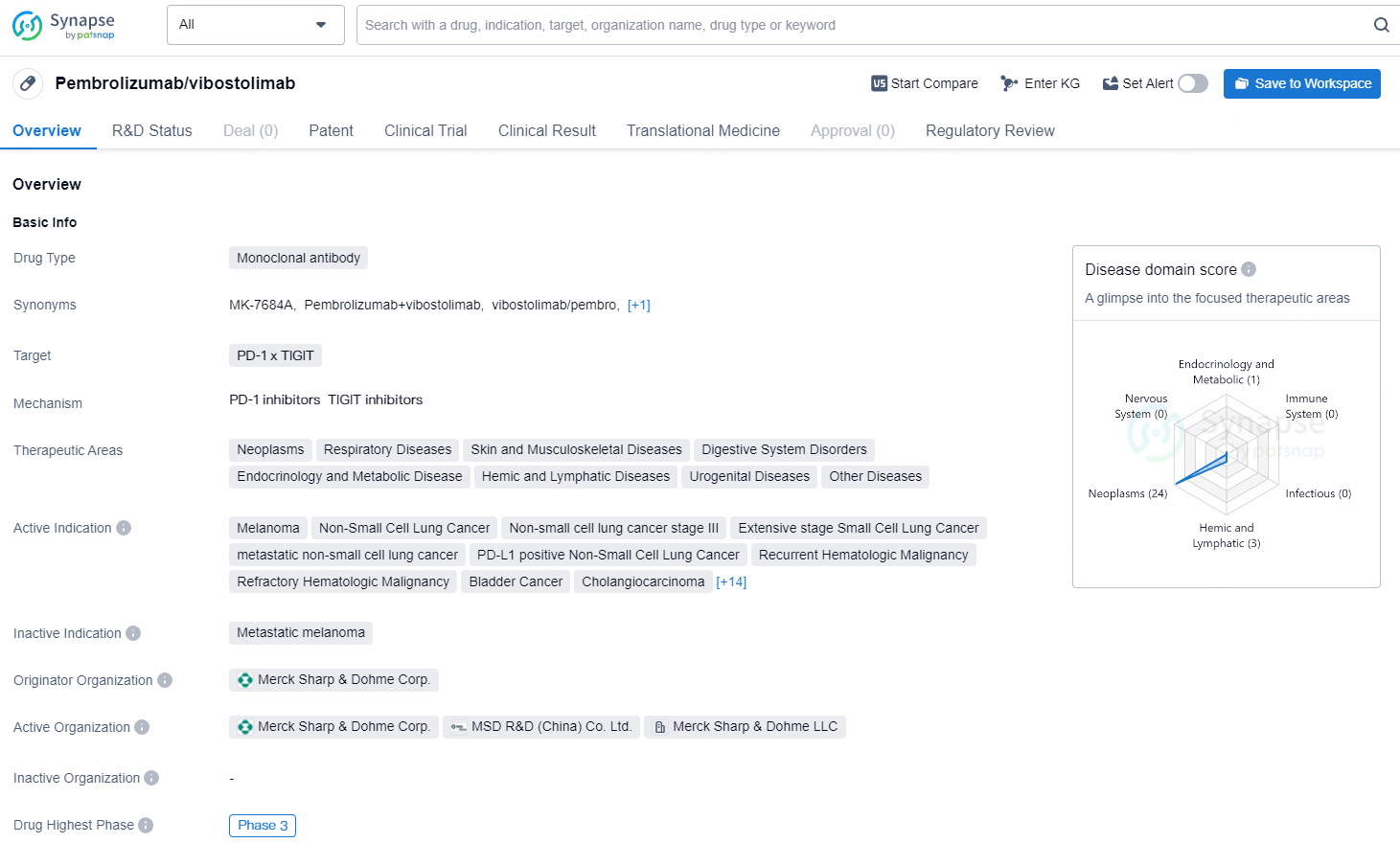
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The ongoing trial is assessing the investigational fixed-dose combination of vibostolimab, an anti-TIGIT antibody, and pembrolizumab (KEYTRUDA ), Merck's anti-PD-1 therapy, alongside chemotherapy, compared to atezolizumab with chemotherapy for initial treatment in extensive-stage small cell lung cancer patients.
In a planned analysis, data revealed that the primary endpoint of overall survival reached the pre-set futility criteria. Furthermore, compared to the control group, patients in the vibostolimab and pembrolizumab combination group experienced a higher incidence of adverse events and immune-related AEs. A thorough analysis of this study remains in progress.
Merck is informing study investigators about the decision and recommending that patients discontinue the fixed-dose combination of vibostolimab and pembrolizumab. Patients are being offered the alternative of treatment with atezolizumab. The findings will be communicated to the scientific community.
Merck has a broad clinical development program targeting lung cancer and is conducting several registration-enabling studies, focusing on earlier disease stages and innovative combinations.
Ongoing Phase 3 trials evaluating the vibostolimab and pembrolizumab fixed-dose combination in lung cancer, which are regularly monitored by external data monitoring committees, include KeyVibe-003, KeyVibe-006, and KeyVibe-007. Interim reviews by safety monitoring committees have not led to any study modifications to date, and the studies continue with comprehensive safety monitoring.
Vibostolimab (MK-7684), an investigational humanized anti-TIGIT antibody discovered and developed by Merck, works by blocking the TIGIT receptor from binding to its ligands (CD112 and CD155), thereby activating T lymphocytes to assist in eliminating tumor cells. The fixed-dose combination of vibostolimab and pembrolizumab is under evaluation across various cancers, including lung cancer, other solid tumors, and hematologic malignancies.
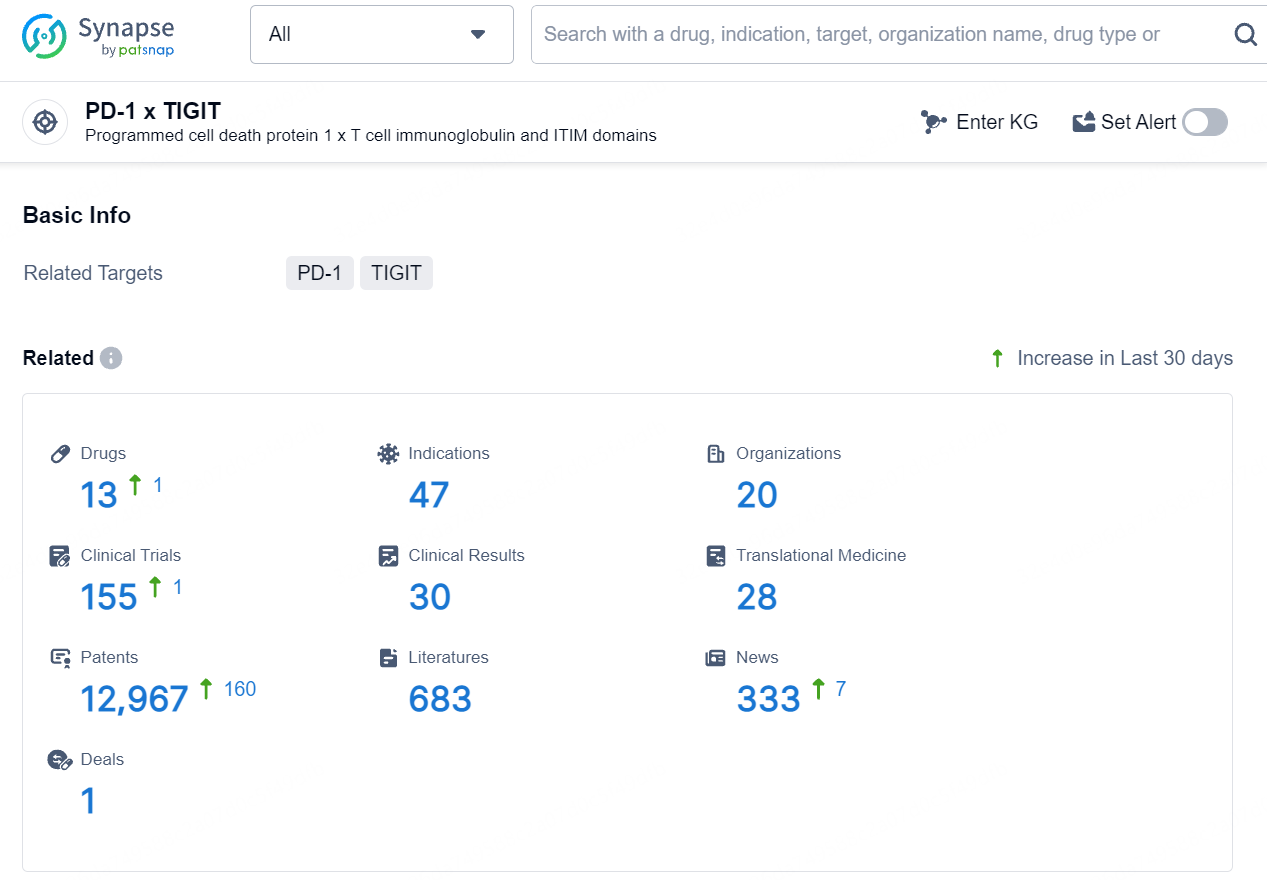
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 12, 2024, there are 13 investigational drugs for the PD-1 and TIGIT targets, including 47 indications, 20 R&D institutions involved, with related clinical trials reaching 155, and as many as 12967 patents.
The potential of Pembrolizumab/vibostolimab in targeting PD-1 and TIGIT and its broad range of active indications in various cancer types highlight its significance in the field of biomedicine. The drug's progression to Phase 3 in both the global and Chinese markets reflects its advanced stage of development and its potential for approval and commercialization in the near future. As an orphan drug, it may also benefit from expedited regulatory processes and market exclusivity upon approval.