Merck's phase III KEYNOTE-A39/EV-302 trial halted disease progression in some new urothelial cancer patients
Merck, which goes by the name MSD outside of North America, has recently publicized promising preliminary results of the KEYNOTE-A39 Phase 3 trial. This trial, executed in partnership with Seagen and Astellas, was examining the efficacy of KEYTRUDA, Merck’s anti-PD-1 treatment, when used in tandem with Padcev (enfortumab vedotin-ejfv), as opposed to chemotherapy in those who have not received prior treatment for locally advanced or metastatic urothelial carcinoma.
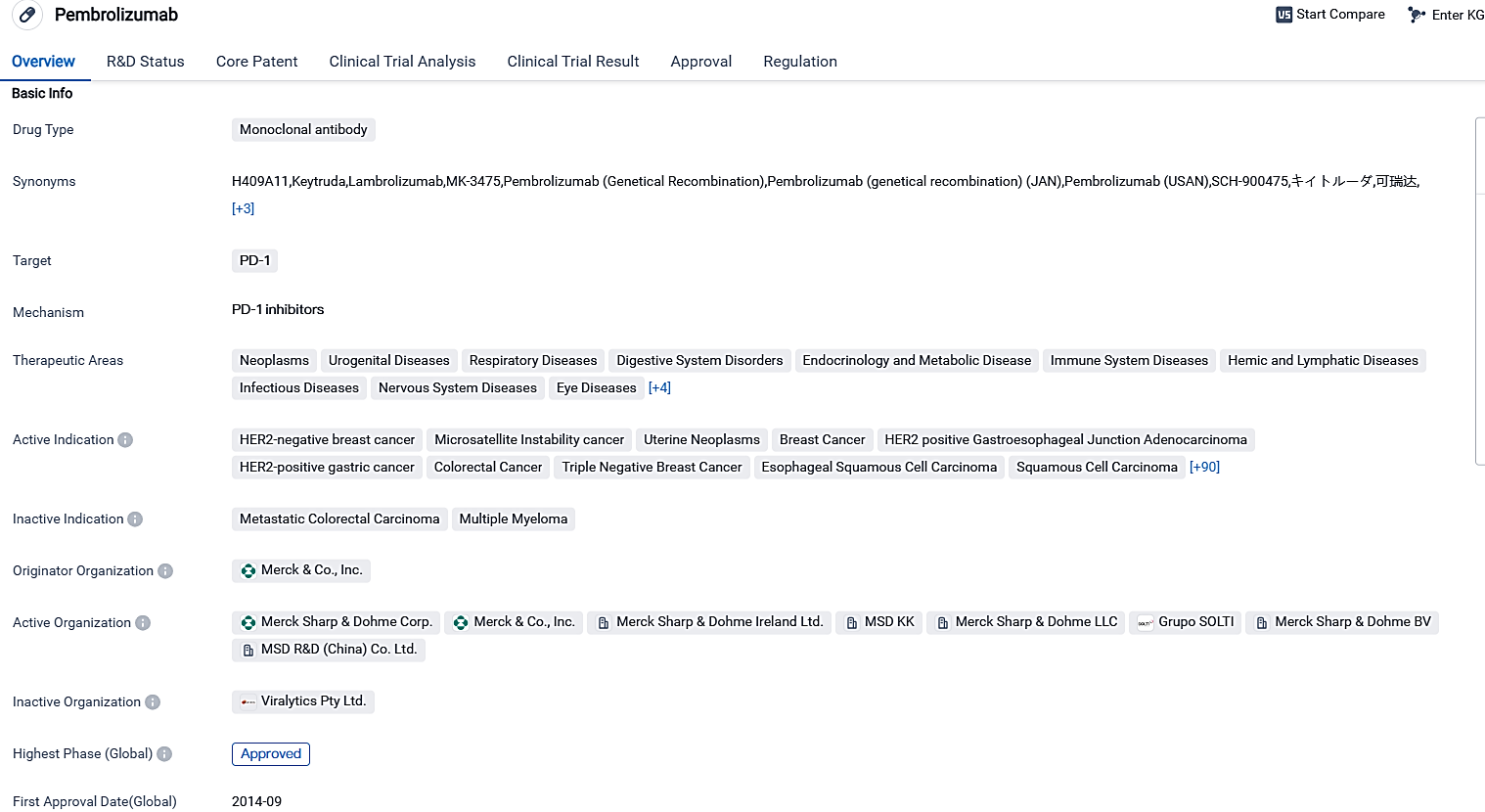
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The experiment incorporated individuals who may be eligible, or otherwise, for cisplatin-based chemotherapy treatment, irrespective of their PD-L1 status. The combination of KEYTRUDA and enfortumab vedotin in the experiment satisfied its dual primary objectives of OS and PFS, revealing a statistically significant and clinically relevant advancement compared to chemotherapy in patients who have not received prior treatment for la/mUC.
Moreover, the combination demonstrated a significant enhancement in ORR, a crucial secondary objective, compared to chemotherapy. The safety characteristics of KEYTRUDA and enfortumab vedotin in this research were in line with previously conducted studies on this combination.
“The outcome of this crucial Phase 3 study is extremely promising and demonstrated a statistically significant survival advantage for the pairing of KEYTRUDA with the antibody-drug conjugate enfortumab vedotin in patients eligible or not for cisplatin who have not received prior treatment for locally advanced or metastatic urothelial carcinoma,” noted Dr. Eliav Barr, senior vice president and chief of global clinical development, along with holding the position of chief medical officer.
He further added, “This is a pivotal progress as numerous patients with advanced urothelial carcinoma continue to experience disease progression post chemotherapy. We are eager to discuss these findings with the medical fraternity as well as the regulatory authorities.”
KEYTRUDA is an anti-programmed death receptor-1 (PD-1) treatment that boosts the body’s immune system's ability to identify and combat cancer cells. KEYTRUDA is a humanized monoclonal antibody that obstructs the interaction between PD-1 and its ligands, PD-L1 and PD-L2, subsequently activating T lymphocytes which could potentially influence both cancer and healthy cells.
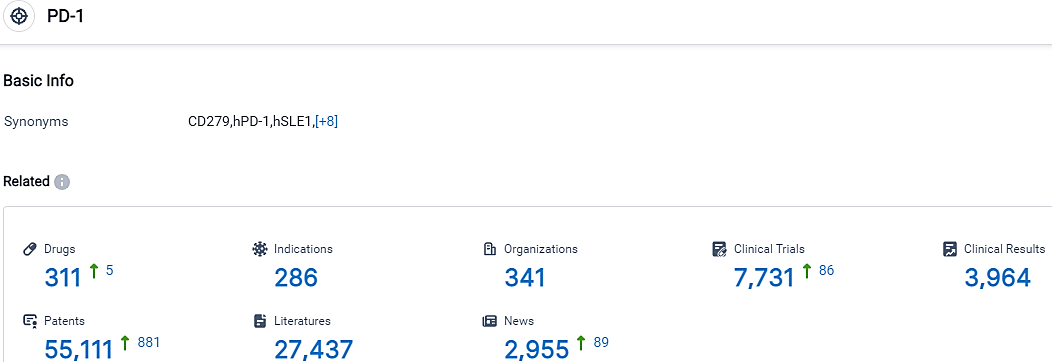
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 29, 2023, there are 311 investigational drugs for the PD-1 target, including 286 indications,341 R&D institutions involved, with related clinical trials reaching 7731,and as many as 55111 patents.
KEYTRUDA is a type of monoclonal antibody medication designed to act on PD-1, with authorization for employment across a wide spectrum of cancers and other health conditions. Its efficacy has been demonstrated in numerous clinical studies, leading to obtaining regulatory clearance across various nations.