SL-172154: Brief Review of its R&D Progress and the Clinical Result in 2023 ASH
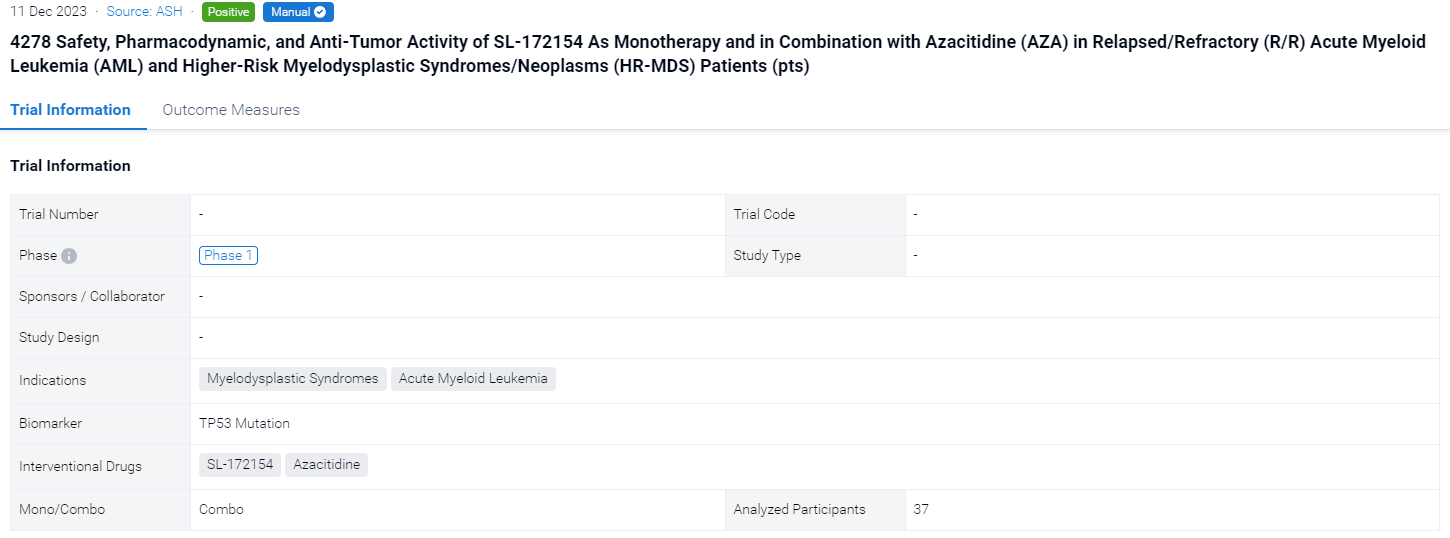
In 2023 ASH, the safety, pharmacodynamic, and anti-tumor activity of SL-172154 as monotherapy and in combination with Azacitidine (AZA) in relapsed/refractory (R/R) acute myeloid leukemia (AML) and higher-risk myelodysplastic syndromes/neoplasms (HR-MDS) patients (pts) will be reported orally.
SL-172154's R&D Progress
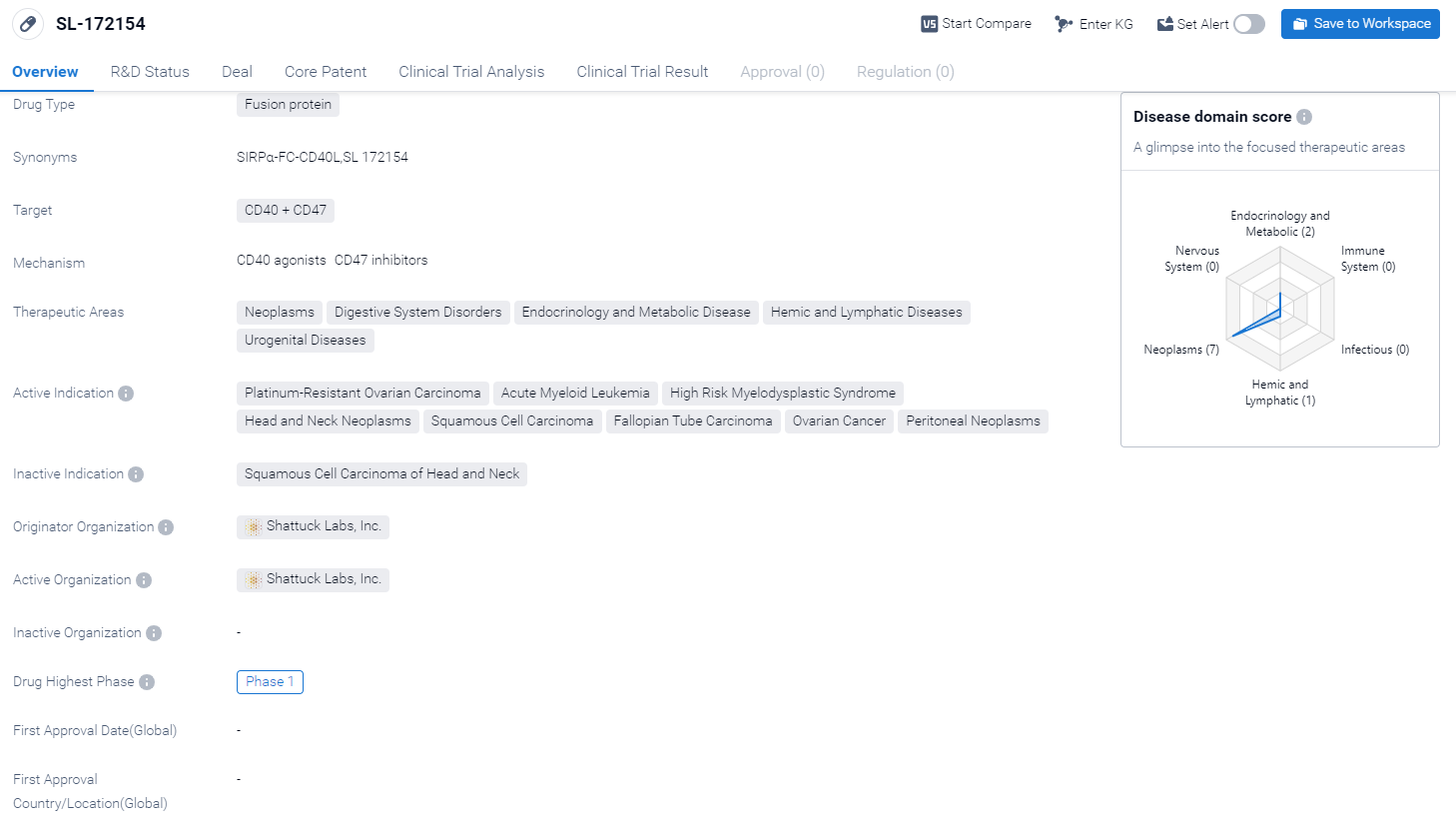
The drug SL-172154 is a fusion protein that targets CD40 and CD47. Fusion proteins are a type of therapeutic agent that combines two or more proteins to create a new molecule with enhanced therapeutic properties. The therapeutic areas targeted by SL-172154 include neoplasms (abnormal growth of cells), digestive system disorders, endocrinology and metabolic diseases, hemic and lymphatic diseases, and urogenital diseases.
Currently, the drug is in Phase 1 of clinical development, which means it is being tested in a small group of patients to evaluate its safety and dosage. And the clinical trial areas for SL-172154 are primarily in the United States, United Kingdom, and Canada. The key indication is Acute Myeloid Leukemia and Myelodysplastic Syndromes. .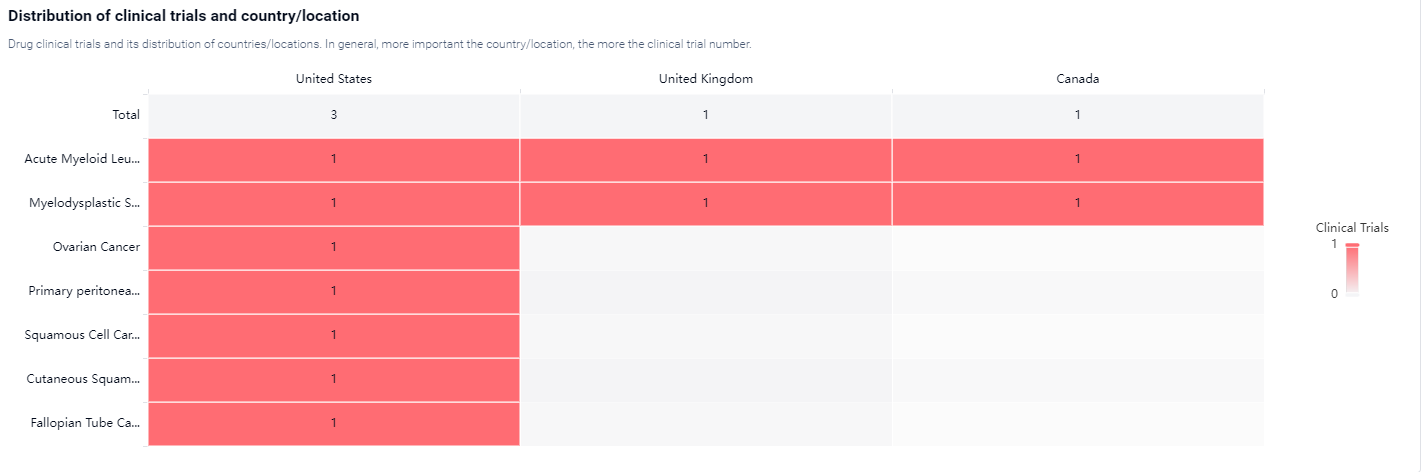
Detailed Clinical Outcome of SL-172154
The objectives of this dose escalation cohorts include evaluation of safety, dose-limiting toxicity (DLT), recommended phase 2 dose, pharmacokinetic and pharmacodynamic effects and efficacy per IWG 2006 for MDS and ELN 2017 for AML.
In this study, SL-172154 was administered IV weekly of a 28-day cycle as monotherapy or with AZA 75 mg/m2 for 7 days per cycle. mTPI-2 was used for dose escalation with a DLT threshold of 20%. Pts ≥ 18 years old with R/R HR-MDS or R/R AML were eligible for dose escalation cohorts. In addition, UnTx pts with TP53 mutant (TP53m)-MDS were eligible for SL-AZA cohorts.
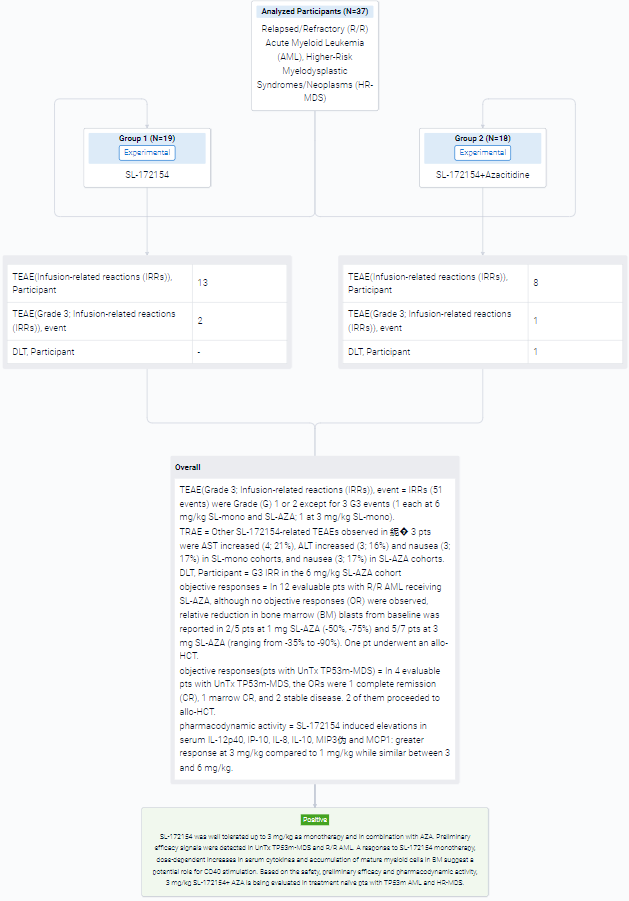
The result showed that Infusion-related reactions (IRRs) were the most common SL-172154-related treatment-emergent AEs (TEAEs) reported in 13 (68%) and 8 (44%) pts in SL-mono and SL-AZA cohorts, respectively. IRRs (51 events) were Grade (G) 1 or 2 except for 3 G3 events (1 each at 6 mg/kg SL-mono and SL-AZA; 1 at 3 mg/kg SL-mono). Other SL-172154-related TEAEs observed in ≥ 3 pts were AST increased (4; 21%), ALT increased (3; 16%) and nausea (3; 17%) in SL-mono cohorts, and nausea (3; 17%) in SL-AZA cohorts. All events of AST/ALT increased were transient. Only one DLT (G3 IRR in the 6 mg/kg SL-AZA cohort) was reported, which required a dose reduction to 3 mg/kg. As of July 10, 2023, in SL-mono cohorts, 1 pt with R/R AML post 7+3, FLAG and VEN/AZA achieved Morphologic Leukemia-Free State (blast reduction from 19% to <5%) after 1 cycle of 6 mg/kg SL-172154. In 12 evaluable pts with R/R AML receiving SL-AZA, although no objective responses (OR) were observed, relative reduction in bone marrow (BM) blasts from baseline was reported in 2/5 pts at 1 mg SL-AZA (-50%, -75%) and 5/7 pts at 3 mg SL-AZA (ranging from -35% to -90%). SL-172154 induced elevations in serum IL-12p40, IP-10, IL-8, IL-10, MIP3α and MCP1: greater response at 3 mg/kg compared to 1 mg/kg while similar between 3 and 6 mg/kg.
It can be concluded that SL-172154 was well tolerated up to 3 mg/kg as monotherapy and in combination with AZA. Preliminary efficacy signals were detected in UnTx TP53m-MDS and R/R AML. A response to SL-172154 monotherapy, dose-dependent increases in serum cytokines and accumulation of mature myeloid cells in BM suggest a potential role for CD40 stimulation. Based on the safety, preliminary efficacy and pharmacodynamic activity, 3 mg/kg SL-172154+ AZA is being evaluated in treatment naïve pts with TP53m AML and HR-MDS.
How to Easily View the Clinical Results Using Synapse Database?
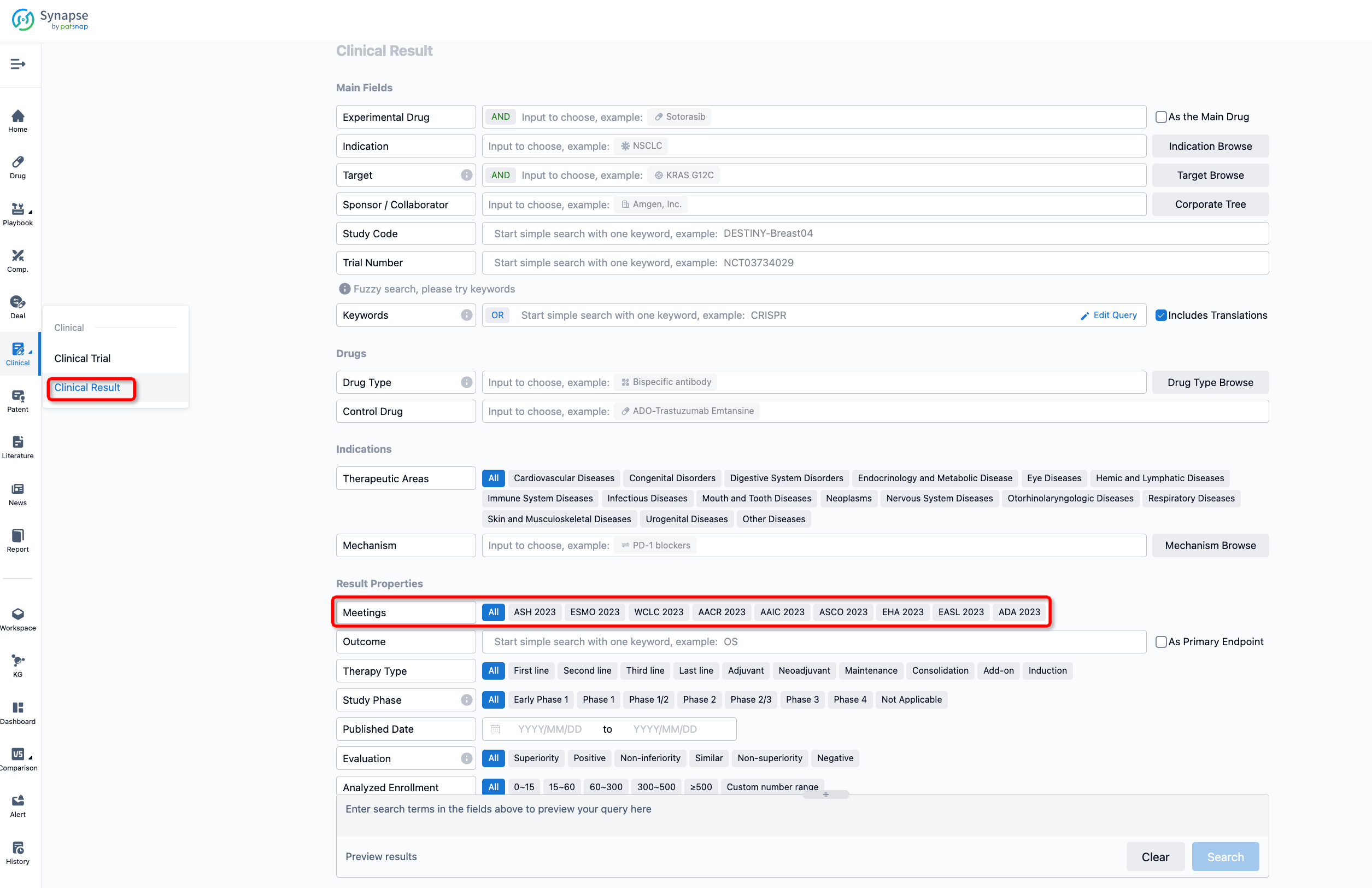
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
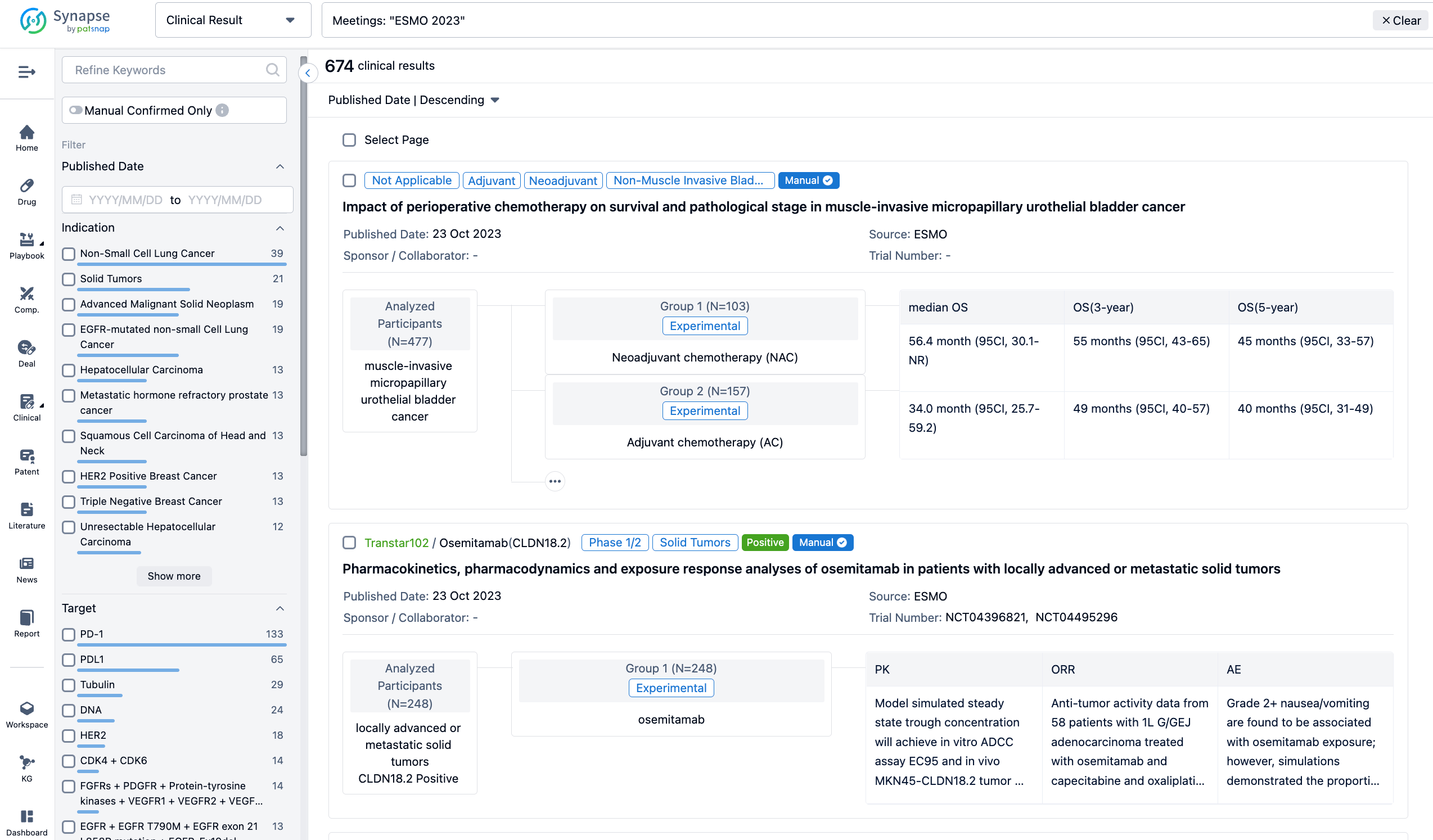
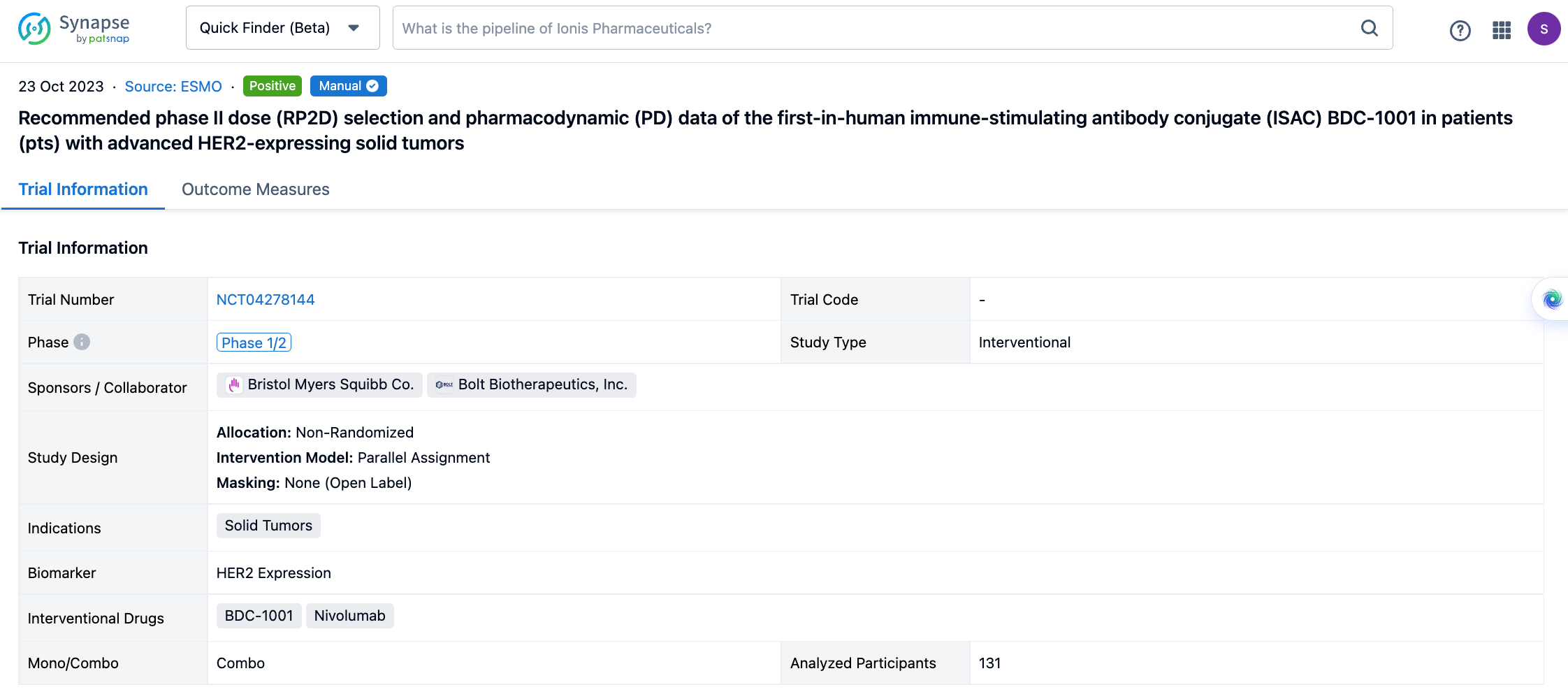
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
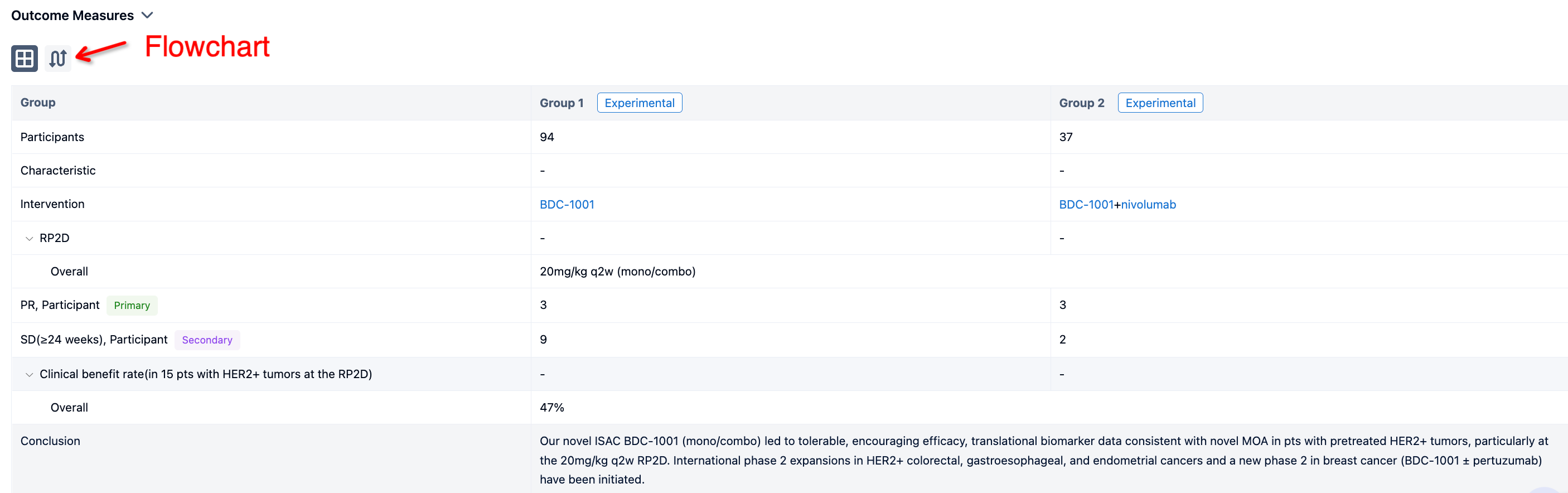
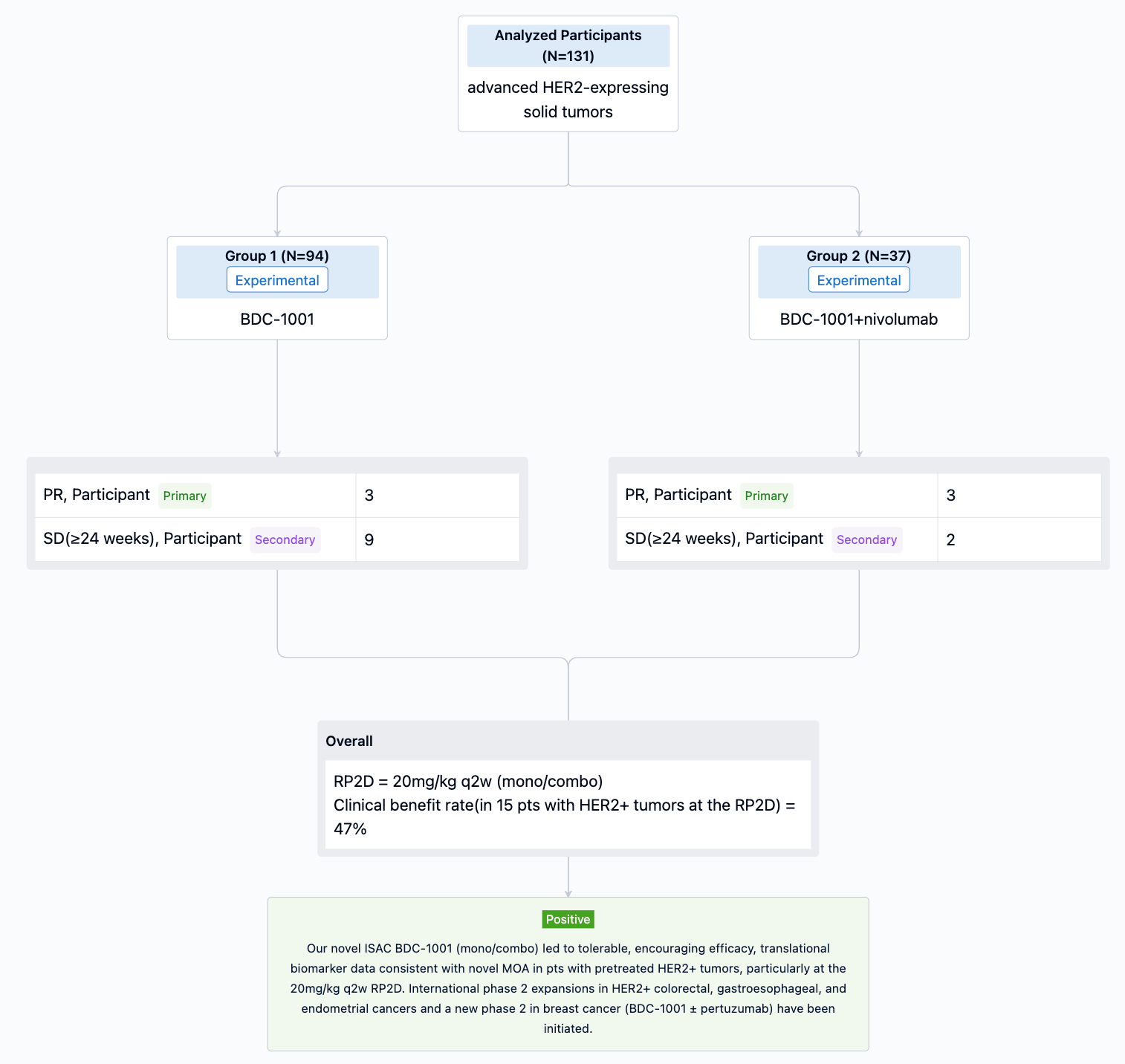
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
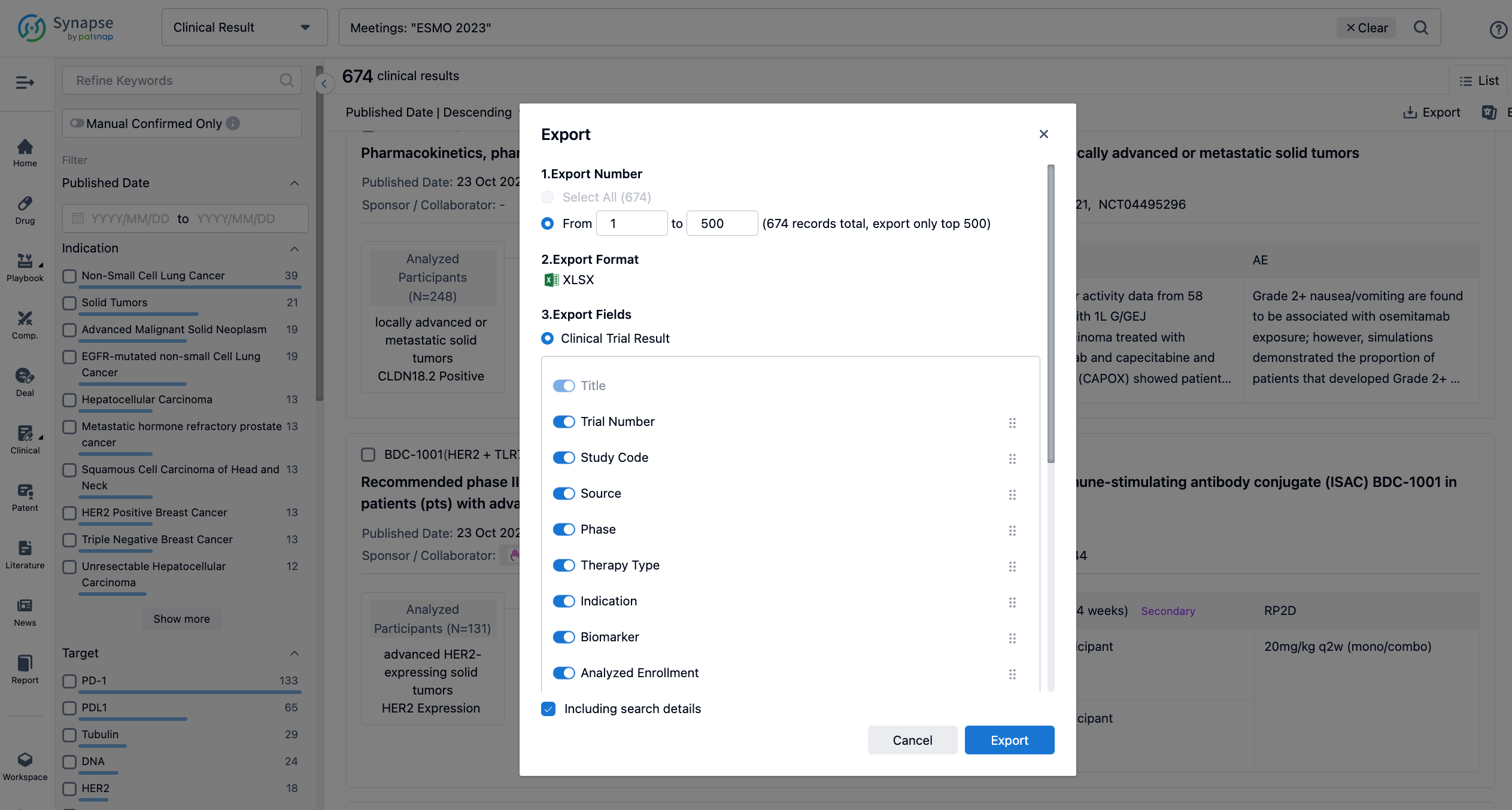
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!