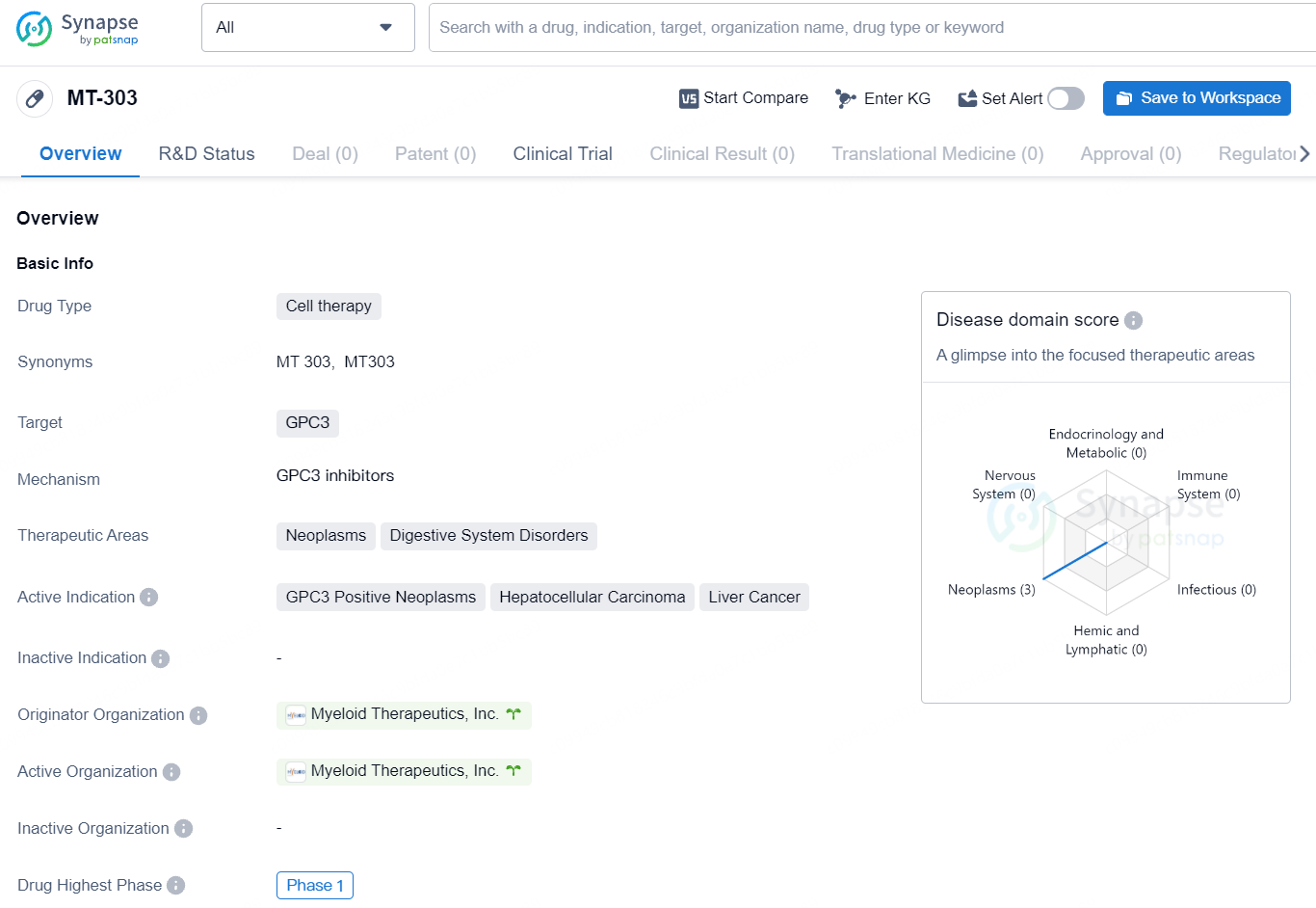
MT-303: Myeloid Therapeutics Launches Phase 1 HCC Trial with RNA CAR Targeting GPC3
Myeloid Therapeutics, Inc., an immunology company at the clinical stage, focusing on RNA therapeutics to defeat cancer, has administered the first dose of MT-303 in a Phase 1 trial for hepatocellular carcinoma. This marks the second in vivo mRNA CAR initiative by Myeloid to advance to clinical trials from its array of in vivo immune cell programming therapies. The administration of MT-303 signifies an important step forward in delivering advanced, novel treatments to patients with liver cancer.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Dr. Matthew Maurer, Myeloid’s Chief Medical Officer, remarked, “We are excited to have rapidly progressed MT-303 into clinical trials as the first in vivo CAR therapy suitable for most liver cancers and other tumors expressing GPC3. MT-303 is administered as a standard off-the-shelf intravenous treatment, without the necessity for preconditioning, and has the potential to initiate a comprehensive immune response against the cancer, enhanced and sustained through ongoing repeated doses.”
GPC3 is a highly attractive target globally due to its abundant expression in hepatocellular carcinoma (HCC) and minimal presence in normal tissues. Unlike autologous cell therapies, Myeloid’s strategy focuses on in vivo programming of immune cells utilizing off-the-shelf mRNA-encoded CAR technology that specifically activates within myeloid cells. MT-303 equips myeloid cells with a unique chimeric antigen receptor that enables these cells to eliminate hepatocellular carcinoma and activate an adaptive immune response against the tumor. This synchronized immune response is critical for establishing long-term immune surveillance and defense against tumor recurrence.
Dr. Timothy Humphries, the lead principal investigator from Linear Clinical Research for the MT-303 trial, stated, “Hepatocellular carcinoma is a highly fatal cancer with few effective treatment options. I am delighted to offer the potential of MT-303, the first in vivo CAR therapy for this condition, to my patients with the hope of achieving tolerable and lasting clinical benefits.”
Daniel Getts, Ph.D., CEO of Myeloid, added, “The Myeloid team is continuously advancing and revolutionizing cancer treatment with the world’s first clinical-stage in vivo mRNA CAR therapies. We have multiple ongoing clinical trials assessing our therapeutic candidates, including MT-302, our innovative TROP2-targeting mRNA CAR in dose escalation studies, and now adding MT-303. This demonstrates our proven ability to translate advanced mRNA CAR technologies into clinical candidates.”
Dr. Getts continued, “Strategically, these clinical programs offer insights as we broaden our development portfolio to target other markers, different immune cell types, and novel receptor combinations for our in vivo CAR methodologies.”
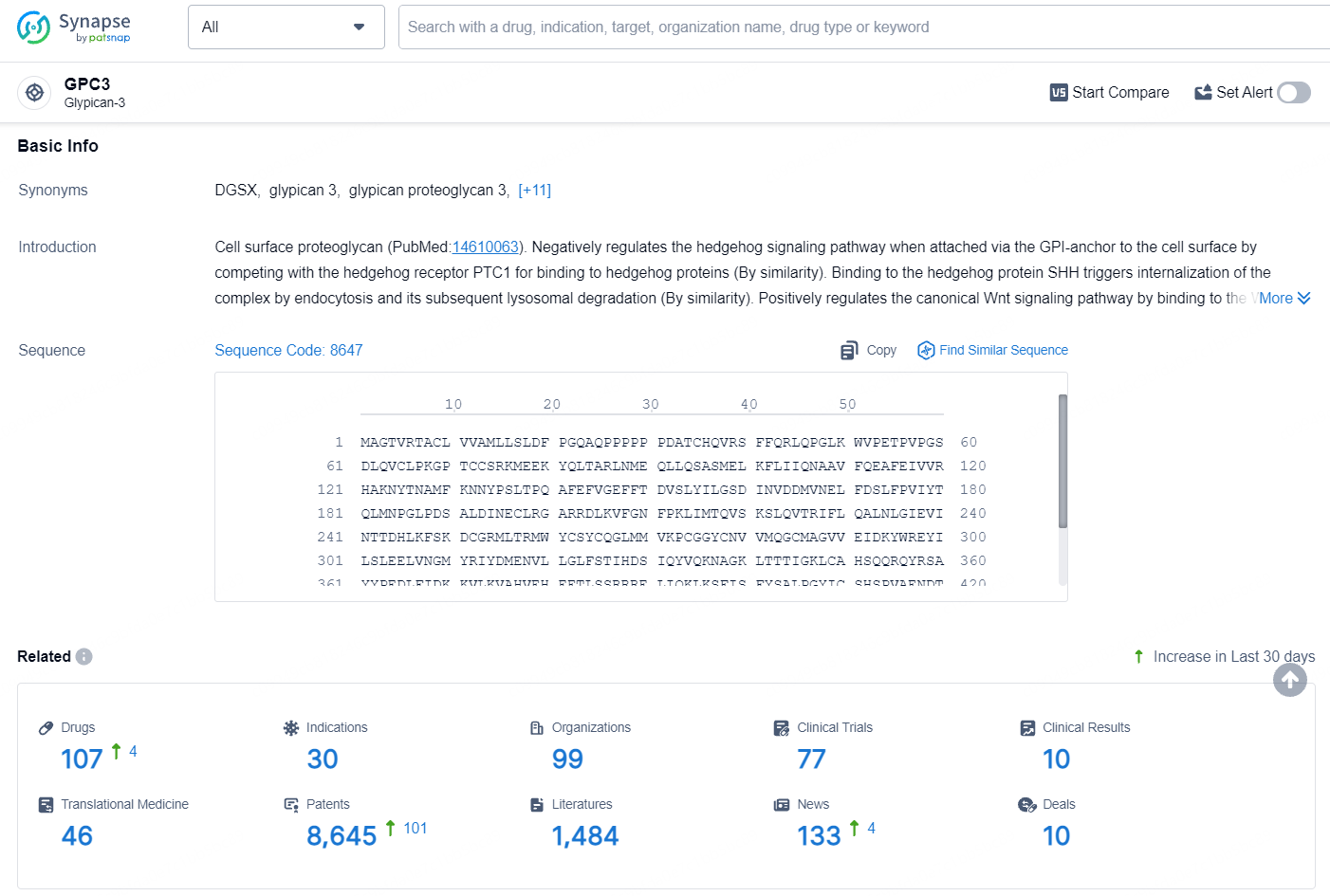
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 6, 2024, there are 107 investigational drugs for the GPC3 target, including 30 indications, 99 R&D institutions involved, with related clinical trials reaching 77, and as many as 8645 patents.
MT-303 represents the first candidate in a new therapeutic modality targeting hepatocellular carcinoma. This clinical candidate is a first-in-class, GPC3-FcA-LNP, with a strong preclinical profile supporting its advance into this first-in-human trial. GPC3 is overexpressed in most human hepatocellular carcinomas, with limited expression in corresponding normal tissues. MT-303 has demonstrated strong expression in myeloid cells and a favorable safety profile in rodents and non-human primates. Unlike other therapies, MT-303 brings the potent