Is Lotilaner approved by the FDA?
Lotilaner ophthalmic, marketed under the brand name Xdemvy, has been approved by the FDA for the treatment of Demodex blepharitis, an eyelid infection caused by Demodex mites, in adults. This approval was granted on July 24, 2023.
Uses
Lotilaner ophthalmic is specifically indicated for treating Demodex blepharitis. This condition is caused by the infestation of Demodex mites, leading to symptoms like inflammation, redness, and discomfort of the eyelids.
Administration and Dosage
- Dosage: Instill one drop in each eye twice daily, approximately 12 hours apart.
- Duration: The recommended treatment period is six weeks.
- Instructions:
- Wash hands before using the eye drops.
- Avoid using the drops while wearing soft contact lenses, as the preservative in the solution could stain the lenses. Wait at least 15 minutes after application before reinserting contact lenses.
- Apply the eye drops by pulling down the lower eyelid to create a small pocket and squeezing a drop into this pocket. Close eyes for 1-2 minutes to allow absorption.
- Ensure not to touch the dropper tip to any surface, including the eye, to avoid contamination.
- Store the medication at room temperature in an upright position and tightly closed. Do not freeze.
Side Effects
- Common side effects: Stinging or burning sensation after application.
- Serious side effects: Signs of eye infection (swelling, redness, severe discomfort, crusting or drainage), eye injury, and vision changes. Immediate medical attention is advised if these occur.
Warnings and Precautions
- Use the medication strictly as directed by a healthcare provider.
- Inform your doctor about any other medications you are taking, as well as any medical conditions or allergies.
- Discuss with your doctor if you are pregnant or breastfeeding.
Storage and Handling
- Store at room temperature.
- Keep the bottle in an upright position and tightly closed when not in use.
- Do not freeze the medication.
Drug Interactions
Lotilaner ophthalmic is not likely to be affected by other drugs due to its local administration in the eyes. However, it is essential to inform your healthcare provider about all the medications you are currently taking, including prescription, over-the-counter, vitamins, and herbal products.
Summary
Lotilaner ophthalmic, approved by the FDA on July 24, 2023, is an effective treatment for Demodex blepharitis. It is important to follow the prescribed dosage and administration instructions to ensure the best therapeutic outcomes while minimizing potential side effects. Always consult with your healthcare provider for personalized medical advice and before starting any new treatment.
How to obtain the latest development progress of all drugs?
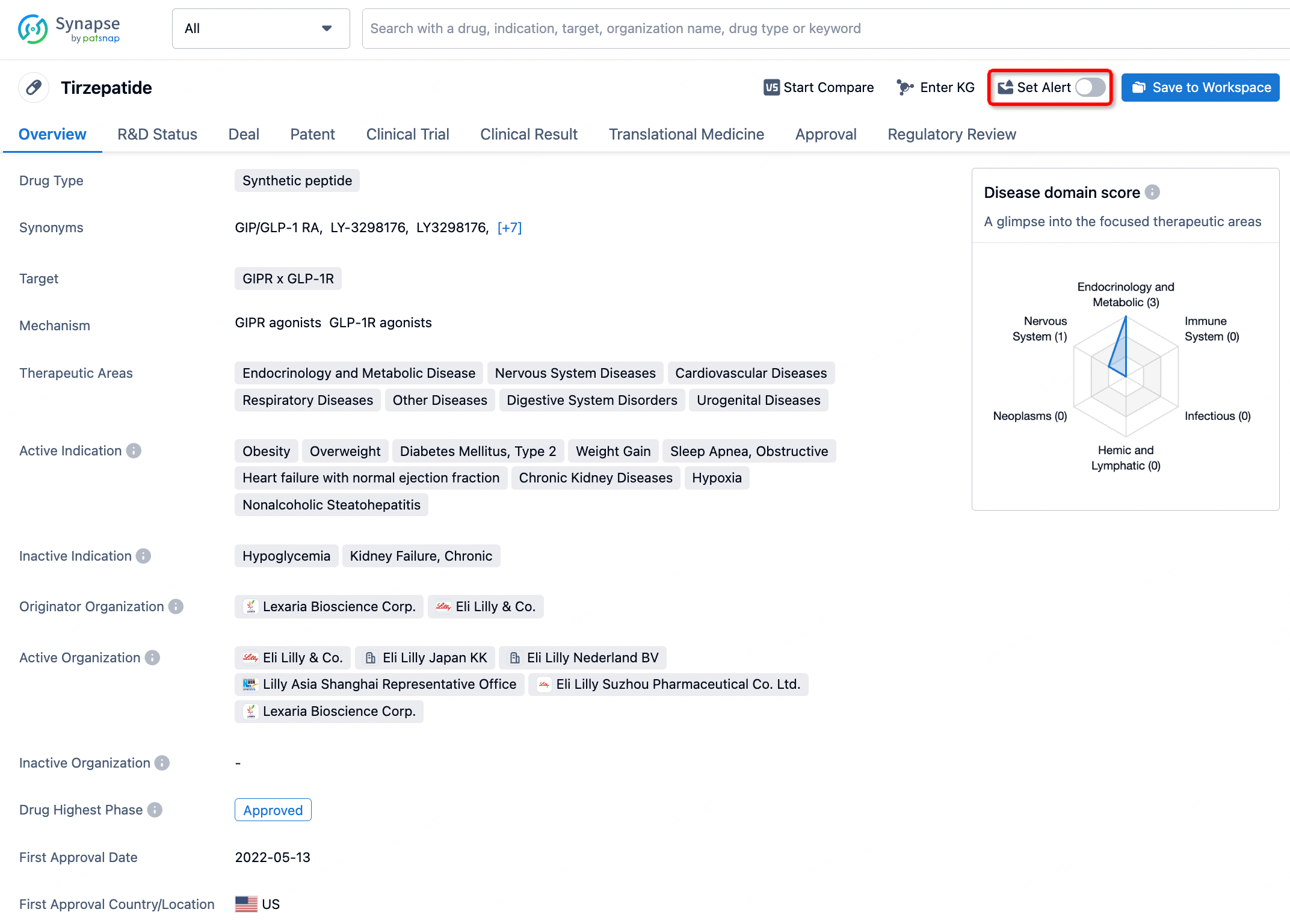
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!