BioNTech Reports Positive Phase 2 Outcomes for mRNA Therapy BNT111 in Advanced Melanoma Patients
BioNTech SE revealed favorable preliminary results from the current Phase 2 clinical study involving individuals with unresectable stage III or IV melanoma whose condition had worsened after receiving anti-PD-(L)1 treatment.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The randomized trial investigates the clinical effectiveness and safety of the novel mRNA cancer therapy BNT111 when used in conjunction with Libtayo (cemiplimab), an anti-PD-1 monoclonal antibody from Regeneron, and also evaluates the effectiveness of each agent alone.
The primary efficacy endpoint of the trial was achieved, showing a significant increase in ORR for patients treated with the combination of BNT111 and cemiplimab, compared to historical controls for the same condition and treatment parameters. Both single-agent arms showed clinical effectiveness. The ORR for cemiplimab monotherapy aligned with the historical control data for anti-PD-(L)1 or anti-CTLA-4 treatments in these patients.
The combination therapy was well tolerated, and the safety profile of BNT111 with cemiplimab was consistent with previous trials of BNT111 with anti-PD-(L)1-based treatments. The Phase 2 trial will proceed as planned to assess the secondary endpoints, which were immature at the time of the primary analysis.
"These Phase 2 results are a critical advancement towards our goal of personalized cancer medicine. We see mRNA as central to future cancer treatments, addressing unmet needs such as anti-PD-(L)1 refractory or resistant melanoma," noted Prof. Özlem Türeci, M.D., Chief Medical Officer and Co-Founder at BioNTech.
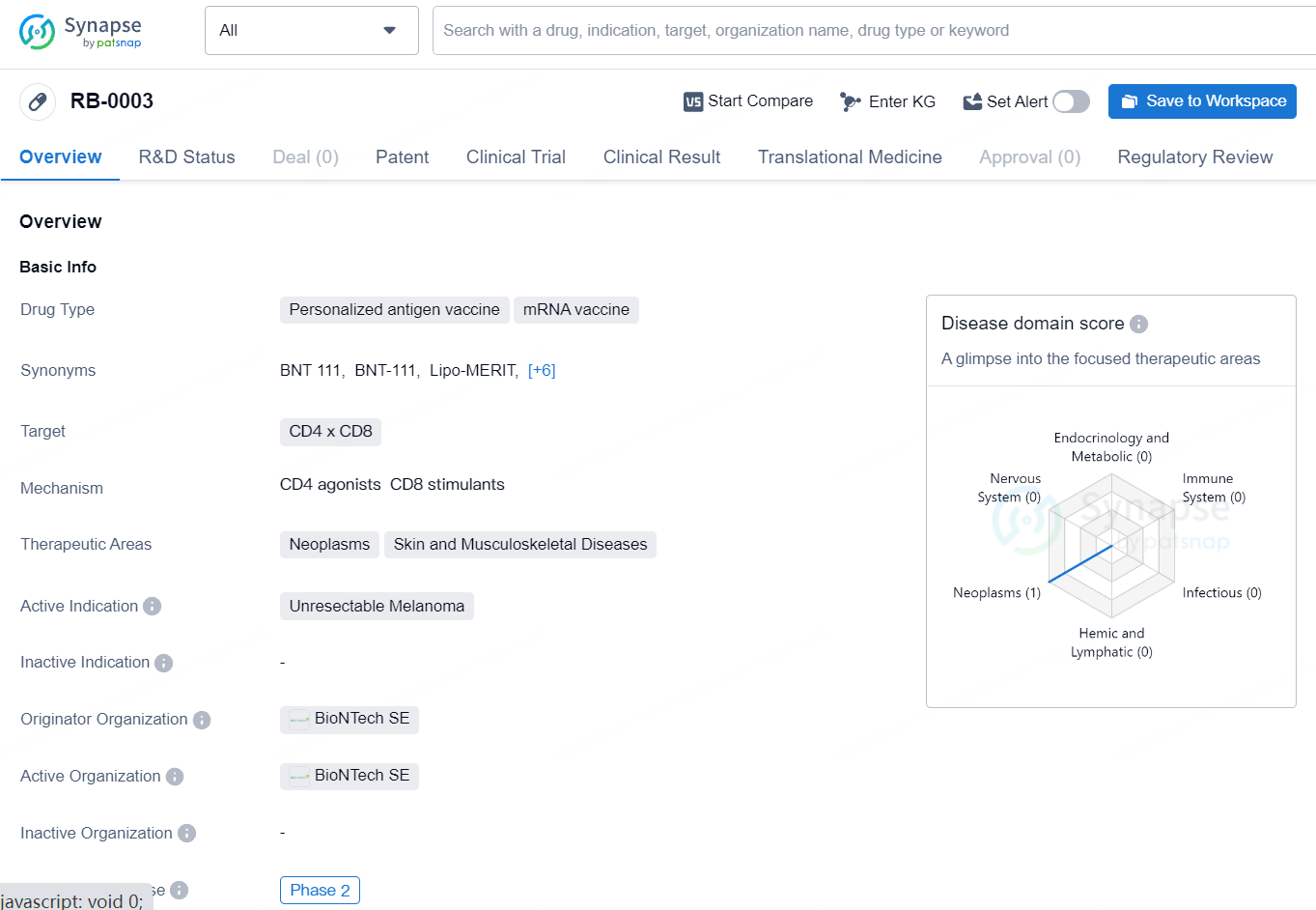
In 2021, the FDA granted Fast Track designation to the combination of BNT111 and cemiplimab for treating anti-PD-1-refractory/relapsed, unresectable Stage III or IV melanoma. The same year, BNT111 also received Orphan Drug designation from the FDA for treating stage IIB through IV melanoma.
BNT111 is an mRNA-based, off-the-shelf cancer immunotherapy designed for intravenous use and encodes a set of four fixed, non-mutated melanoma-associated antigens in a uridine mRNA-lipoplex formulation. Over 90% of patients with cutaneous melanomas express at least one of these antigens.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
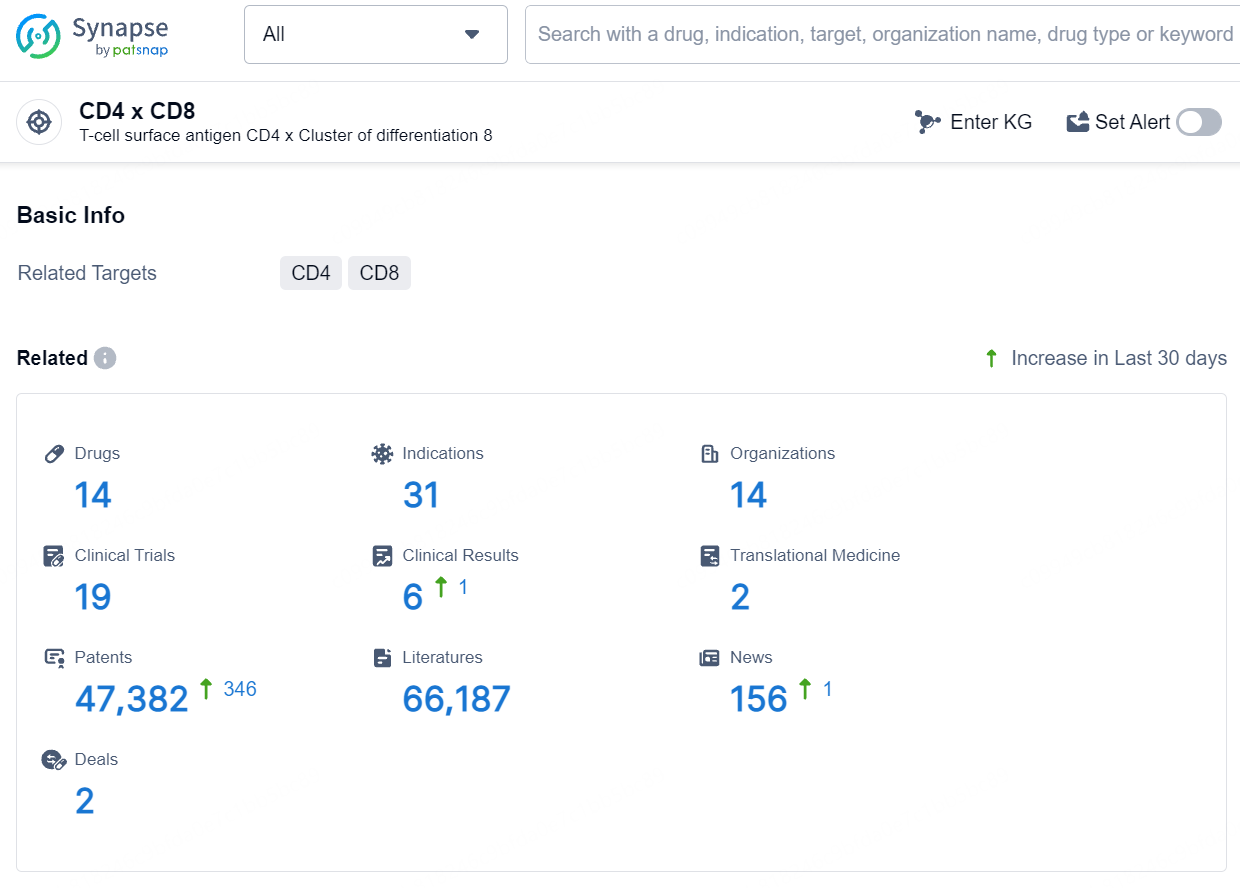
According to the data provided by the Synapse Database, As of August 5, 2024, there are 14 investigational drugs for the CD4 and CD8 targets, including 31 indications, 14 R&D institutions involved, with related clinical trials reaching 19, and as many as 47382 patents.
RB-0003 is a personalized antigen mRNA vaccine, targeting CD4 and CD8 cells for the treatment of unresectable melanoma. The drug has reached Phase 2 of clinical development and has received regulatory designations such as Fast Track and Orphan Drug. Its innovative approach to cancer treatment and potential to address unmet medical needs make RB-0003 a promising candidate in the field of biomedicine.