SanBio's AKUUGO® for Treating Motor Paralysis in Brain Injury Patients: Approval Achieved
SanBio Co., Ltd. announces that on July 31, 2024, it received conditional and time-limited marketing authorization in Japan for its human somatic stem cell-processed product "AKUUGO suspension for intracranial implantation" (INN: vandefitemcel; referred to as "AKUUGO") for the treatment of persistent motor paralysis caused by traumatic brain injury.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Traumatic brain injury (TBI) occurs when a substantial external force, such as from a vehicular accident or a fall, impacts the head, leading to damage in the brain tissue encased within the skull. The onset and type of TBI symptoms can vary greatly among patients. Depending on the specific brain region affected, individuals may exhibit aftereffects such as motor or higher brain dysfunction. Research indicates that brain tissues that are destroyed or damaged do not regenerate on their own, and individuals who progress into the chronic phase of TBI and develop motor paralysis often face prolonged impacts on their everyday and social activities, highlighting a significant medical need.
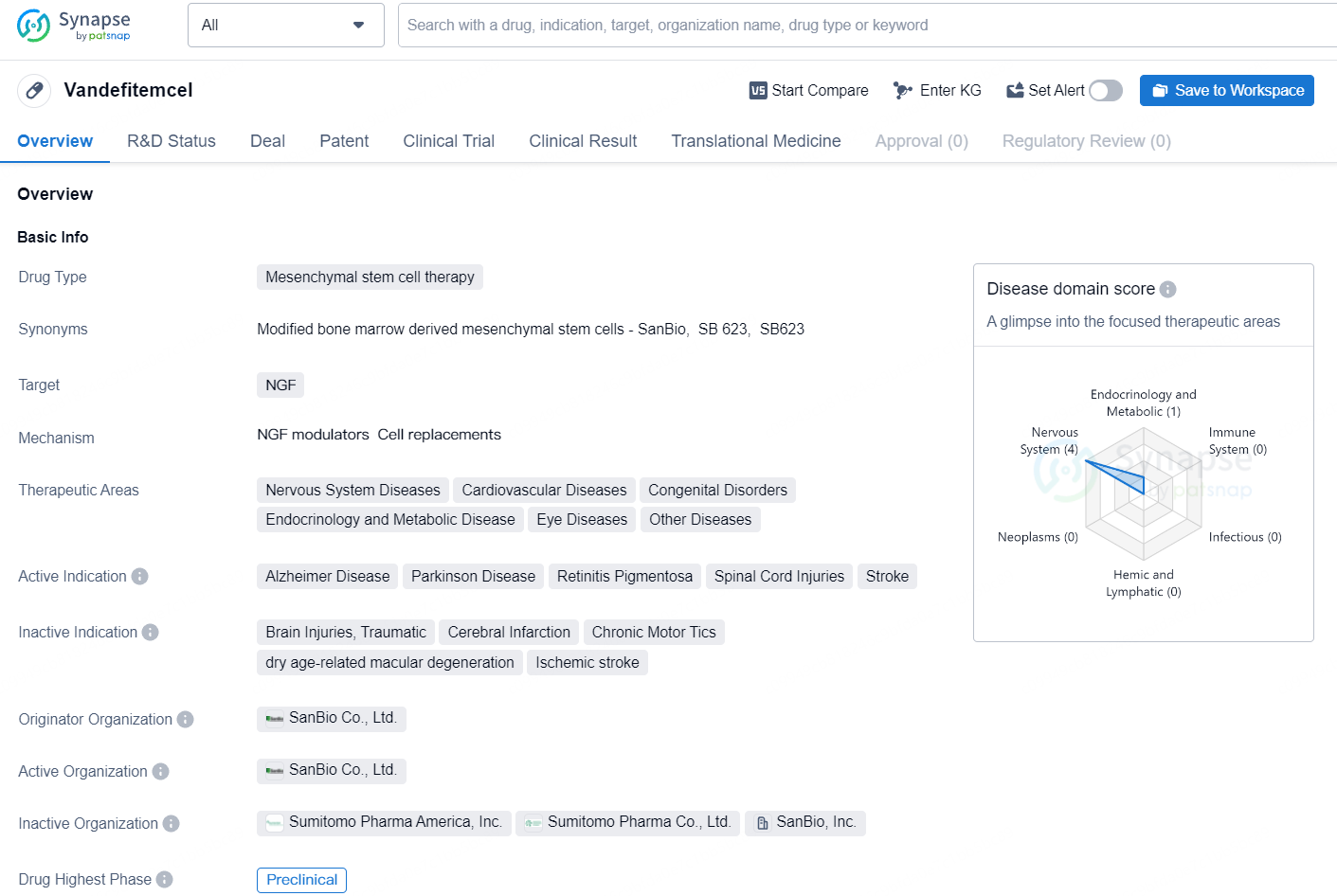
AKUUGO has demonstrated efficacy in mitigating chronic motor paralysis linked to TBI, making it the first and only approved allogeneic cell therapeutic agent globally for this purpose. Additionally, AKUUGO stands out as the pioneering treatment facilitating brain regeneration, and its clinical approval is a landmark achievement.
AKUUGO is produced by culturing mesenchymal stem cells derived from the bone marrow of healthy donors and transiently introducing the human Notch-1 intracellular domain gene into these cultured cells, enhancing their ability to regenerate nerve cells. Transplanting AKUUGO into damaged brain tissues is anticipated to stimulate the release of FGF-2 and other substances, thereby promoting the natural regenerative capacity of impaired nerve cells and encouraging the proliferation and differentiation of new nerve cells. Foundational research also suggests that AKUUGO has neuroprotective benefits, promotes angiogenesis, and exhibits immunomodulatory properties.
"We are thrilled to announce we have received conditional, time-limited approval for AKUUGO after 24 years of dedicated work, allowing us to offer it as a novel treatment for TBI patients in Japan. As a treatment directly administered to the brain, AKUUGO provides a vital and revolutionary option for those with chronic motor paralysis due to TBI. Through AKUUGO, we aspire to bring renewed hope to the lives of numerous TBI patients."
The approval of AKUUGO is grounded in the outcomes of a global Phase 2 clinical trial undertaken by SanBio in both Japan and the United States. Dr. Nobuhito Saito, a Professor of Neurology at the University of Tokyo, led the clinical study and provided the following commentary.®
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
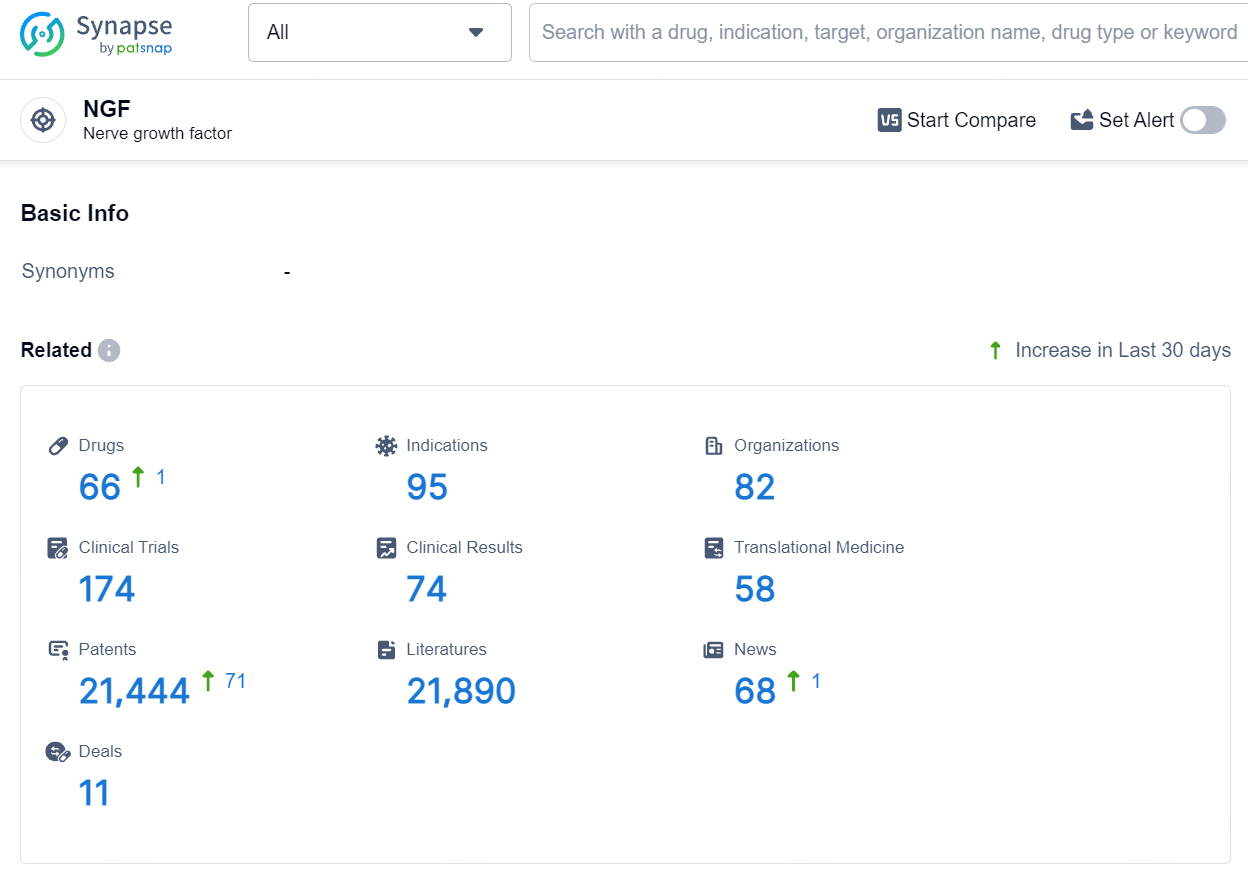
According to the data provided by the Synapse Database, As of August 6, 2024, there are 66 investigational drugs for the NGF target, including 95 indications, 82 R&D institutions involved, with related clinical trials reaching 174, and as many as 21444 patents.
Vandefitemcel is a mesenchymal stem cell therapy targeting NGF with a focus on treating various diseases across different therapeutic areas, particularly in the nervous system and cardiovascular system. Its potential to address conditions such as Alzheimer Disease, Parkinson Disease, and other significant indications makes it a drug of interest in the field of biomedicine. However, further developments and clinical data will be necessary to determine the full scope of its therapeutic potential.