New approach to cancer treatment-MDM2 inhibitors
MDM2 is a crucial protein found in the human body that plays a significant role in regulating cell growth and division. It acts as a negative regulator of the tumor suppressor protein p53, which is responsible for preventing the formation of cancerous cells. MDM2 binds to p53, inhibiting its activity and promoting its degradation, thereby preventing excessive cell growth and maintaining cellular homeostasis. However, when MDM2 is overexpressed or its function is disrupted, it can lead to the inactivation of p53, allowing uncontrolled cell proliferation and potentially contributing to the development of various cancers. Understanding the role of MDM2 is essential for developing targeted therapies to restore p53 function and combat cancer.
MDM2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 20 Sep 2023, there are a total of 57 MDM2 drugs worldwide, from 62 organizations, covering 57 indications, and conducting 144 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.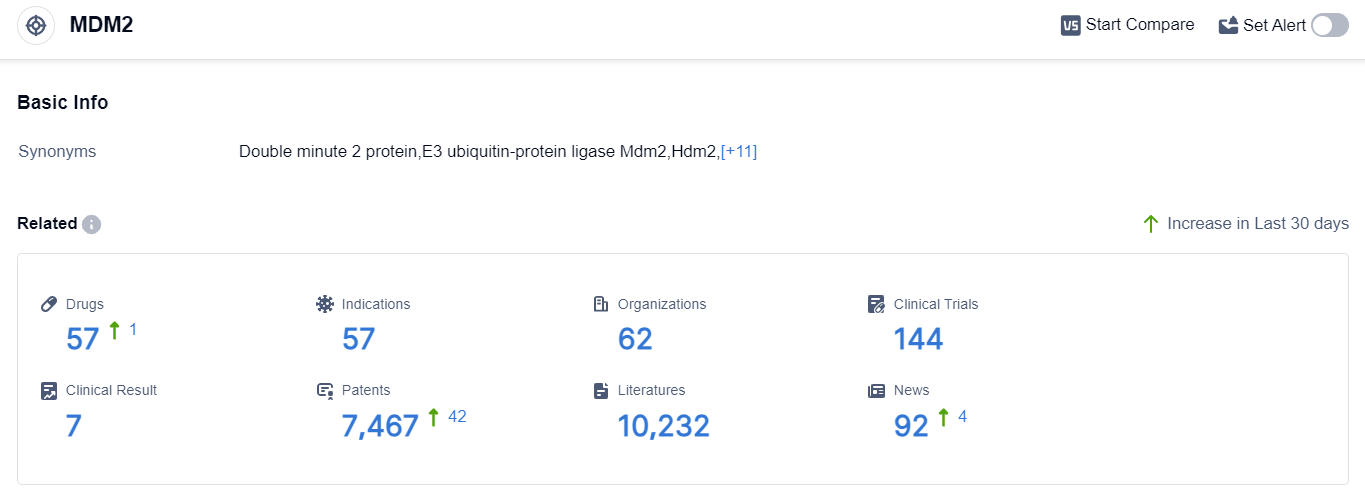
The analysis of the current competitive landscape of target MDM2 reveals that Roche Holding AG, Rain Oncology, Inc., C.H. Boehringer Sohn AG & Co. KG, Kartos Therapeutics, Inc., and Ascentage Pharma Group International Co., Ltd. are the companies growing fastest in this field. These companies have made significant progress in the research and development of drugs targeting MDM2, with Roche Holding AG leading in terms of the highest number of drugs in advanced stages of development.
The approved indications for drugs targeting MDM2 cover a wide range of cancers and hematologic neoplasms, indicating the potential effectiveness of these drugs in treating various diseases. Small molecule drugs, PROTACs, and synthetic peptides are the drug types progressing most rapidly, with intense competition observed in the biosimilar market.
The United States, European Union, and several other countries/locations are actively developing drugs targeting MDM2, indicating a global effort in this field. Further analysis of the research and development institutions involved in biosimilars would provide valuable insights into the competitive landscape. Overall, the target MDM2 shows promising potential for future development in the pharmaceutical industry.
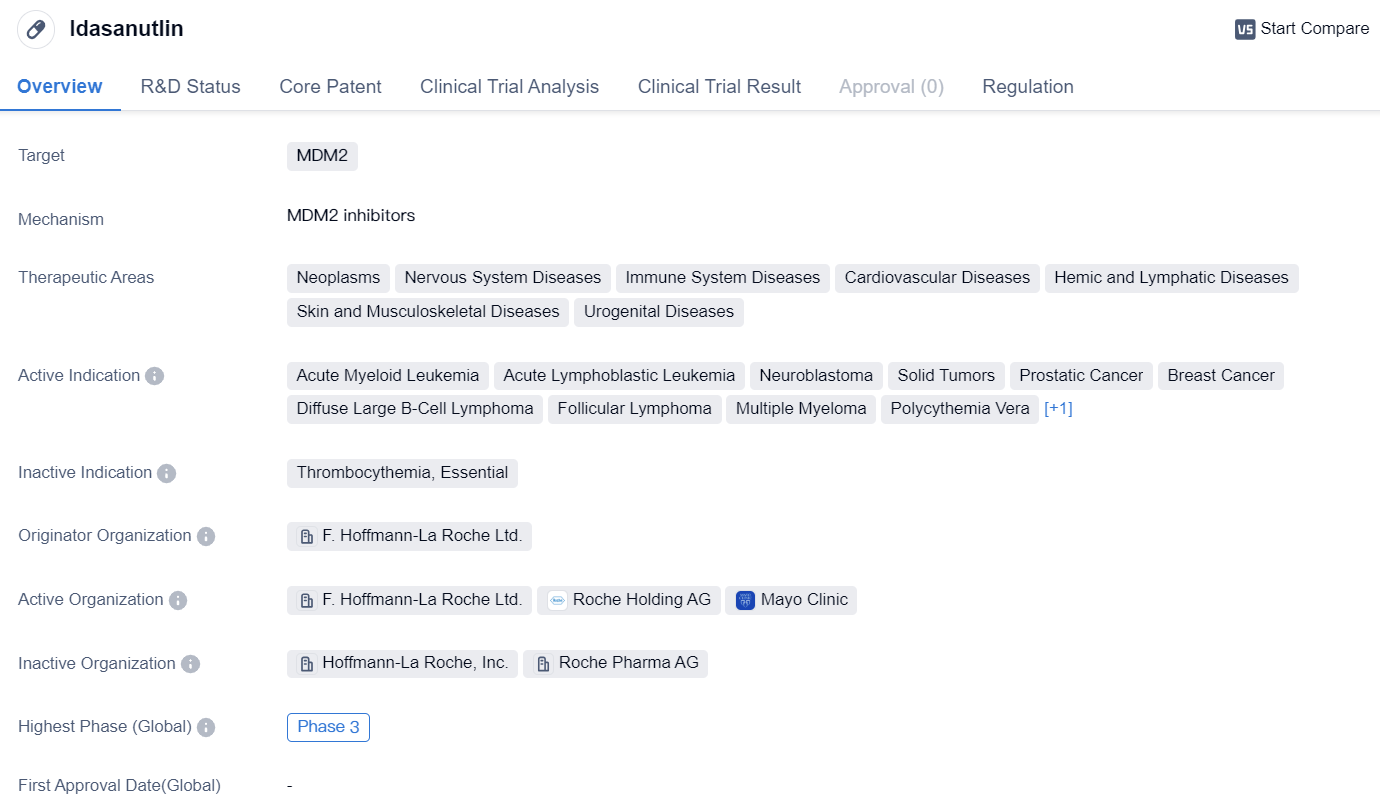
MDM2 inhibitors entering Phase III clinical trials: Idasanutlin
Idasanutlin is a small molecule drug that targets MDM2, a protein that plays a role in regulating the tumor suppressor protein p53. This drug is being developed for the treatment of various therapeutic areas including neoplasms, nervous system diseases, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and urogenital diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of active indications, idasanutlin is being investigated for the treatment of acute myeloid leukemia, acute lymphoblastic leukemia, neuroblastoma, solid tumors, prostatic cancer, breast cancer, diffuse large B-cell lymphoma, follicular lymphoma, multiple myeloma, polycythemia vera, and glioblastoma. These indications cover a wide range of cancers and highlight the potential versatility of idasanutlin in the field of oncology.
The drug is being developed by F. Hoffmann-La Roche Ltd., a well-known pharmaceutical company. It has reached phase 3 of clinical development globally. In China, idasanutlin has reached phase 2, indicating progress in its development in this specific market.
Idasanutlin has been designated as an orphan drug, which means it is intended to treat rare diseases or conditions. This designation provides certain incentives and benefits to the drug developer, such as market exclusivity and financial incentives, to encourage the development of treatments for rare diseases.
In summary, idasanutlin is a small molecule drug that targets MDM2 and is being developed for the treatment of various therapeutic areas, particularly in the field of oncology. It has shown promise in multiple indications, including acute myeloid leukemia, solid tumors, and glioblastoma. With its orphan drug designation and advancement to phase 3 globally, idasanutlin represents a potential treatment option for patients with these diseases.
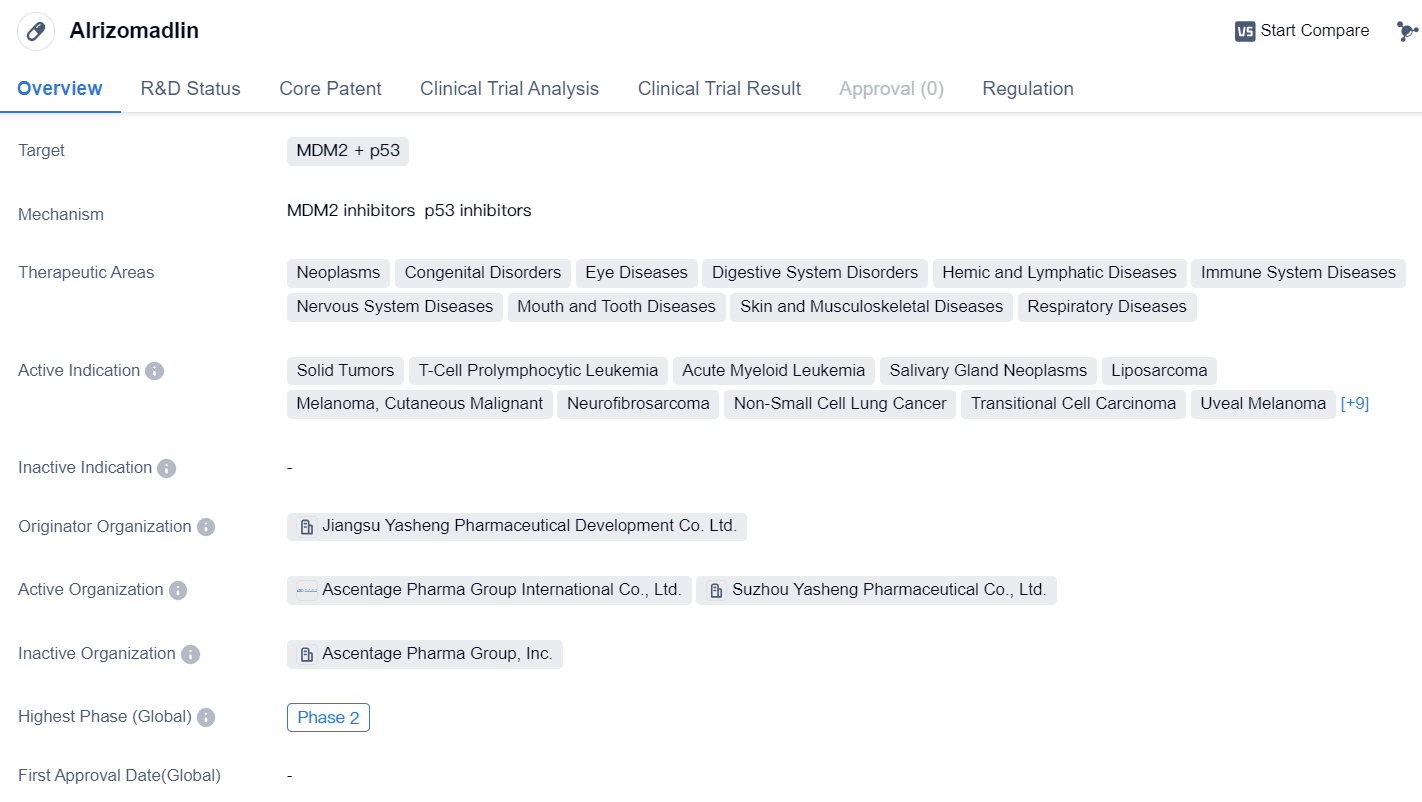
MDM2 inhibitors entering Phase II clinical trials: Alrizomadlin
Alrizomadlin is a small molecule drug that targets MDM2 and p53, making it a potential candidate for the treatment of various diseases. It falls under the therapeutic areas of neoplasms, congenital disorders, eye diseases, digestive system disorders, hemic and lymphatic diseases, immune system diseases, nervous system diseases, mouth and tooth diseases, skin and musculoskeletal diseases, and respiratory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown promise in the treatment of solid tumors, T-cell prolymphocytic leukemia, acute myeloid leukemia, salivary gland neoplasms, liposarcoma, cutaneous malignant melanoma, neurofibrosarcoma, non-small cell lung cancer, transitional cell carcinoma, uveal melanoma, hematologic neoplasms, myelodysplastic syndromes, lymphoma, chronic myelomonocytic leukemia, mixed phenotype acute leukemia, neuroblastoma, dystrophy, macular, stomach cancer, and retinoblastoma.
Alrizomadlin is developed by Jiangsu Yasheng Pharmaceutical Development Co. Ltd., an originator organization in the pharmaceutical industry. Currently, the drug has reached Phase 2 of clinical development in both global and China trials. This indicates that it has progressed through initial safety testing and has shown potential efficacy in treating the targeted diseases.
In terms of regulation, Alrizomadlin has been granted Fast Track designation, which expedites the development and review process by the regulatory authorities. Additionally, it has also received Orphan Drug status, indicating that it is intended to treat rare diseases. Furthermore, the drug has been recognized as a treatment for rare pediatric diseases.
Based on the available information, Alrizomadlin shows promise as a potential therapeutic option for a wide range of diseases. Its small molecule nature and targeting of MDM2 and p53 make it a potentially versatile drug. However, further clinical trials and regulatory approvals are required to establish its safety and efficacy.