Progress of IL-17A Inhibitors in the Treatment of Autoimmune and Inflammatory Diseases
IL-17A, or Interleukin-17A, is a cytokine that plays a crucial role in the human body's immune response. It is primarily produced by T-helper 17 (Th17) cells and other immune cells. IL-17A acts as a pro-inflammatory mediator, promoting the recruitment and activation of immune cells to sites of infection or tissue damage. It is involved in various autoimmune and inflammatory diseases, including psoriasis, rheumatoid arthritis, and inflammatory bowel disease. Understanding the role of IL-17A has led to the development of targeted therapies, such as IL-17A inhibitors, which have shown significant efficacy in treating these conditions by reducing inflammation and alleviating symptoms.
IL-17A Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 20 Sep 2023, there are a total of 47 IL-17A drugs worldwide, from 83 organizations, covering 80 indications, and conducting 656 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.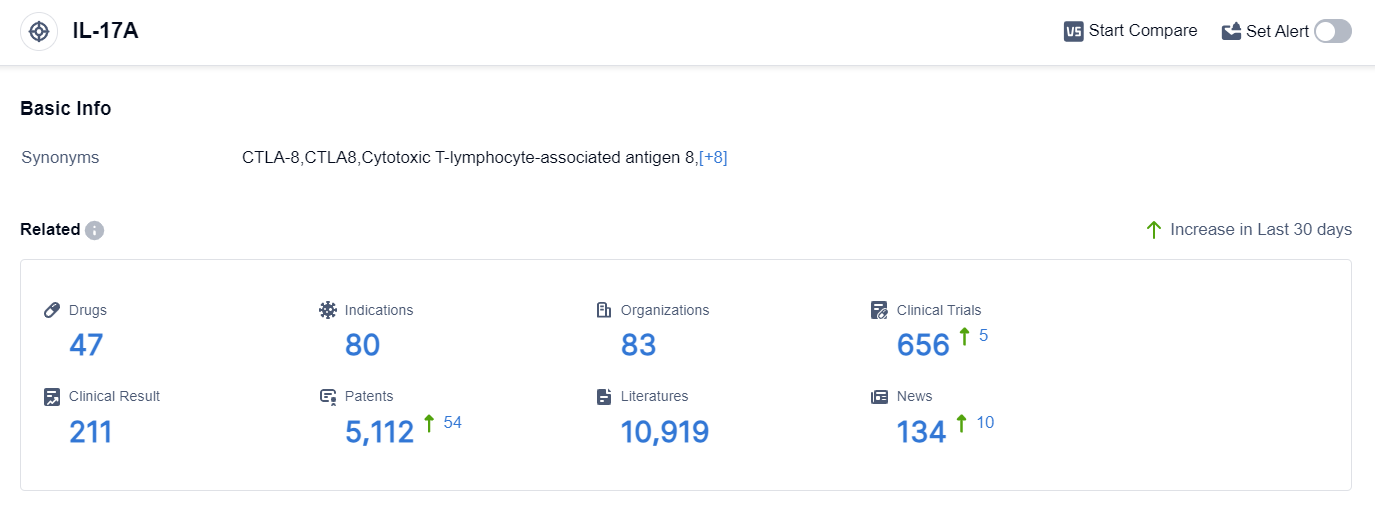
The analysis of the current competitive landscape and future development of target IL-17A reveals that Novartis AG, UCB SA, and Eli Lilly & Co. are the companies growing fastest under this target. These companies have drugs in various stages of development, including approved, NDA/BLA, and different phases of clinical trials.
Drugs targeting IL-17A have been approved for indications such as plaque psoriasis, arthritis, pustular psoriasis, psoriasis vulgaris, hidradenitis suppurativa, spondylarthritis, juvenile arthritis, colorectal cancer, pancreatic cancer, parapsoriasis, Kaposi sarcoma, melanoma, uterine cervical cancer, stomach cancer, and renal cell carcinoma.
Monoclonal antibodies and biosimilars are the drug types progressing most rapidly under the current target IL-17A, indicating intense competition in the market. Research and development institutions involved in the development of biosimilars include Novartis AG, UCB SA, Eli Lilly & Co., Biocad CJSC, Aptose Biosciences, Inc., and Jiangsu Hengrui Pharmaceutical Group Co. Ltd.
China, the United States, Japan, and the European Union are the countries/locations developing fastest under the current target IL-17A. China has shown significant progress in the development of drugs targeting IL-17A.
Overall, the target IL-17A presents a competitive landscape with multiple companies, various indications, and different drug types. The future development of IL-17A drugs will continue to focus on clinical trials, regulatory approvals, and the introduction of innovative therapies to address unmet medical needs.
The world's first fully human IL-17A inhibitor: Secukinumab
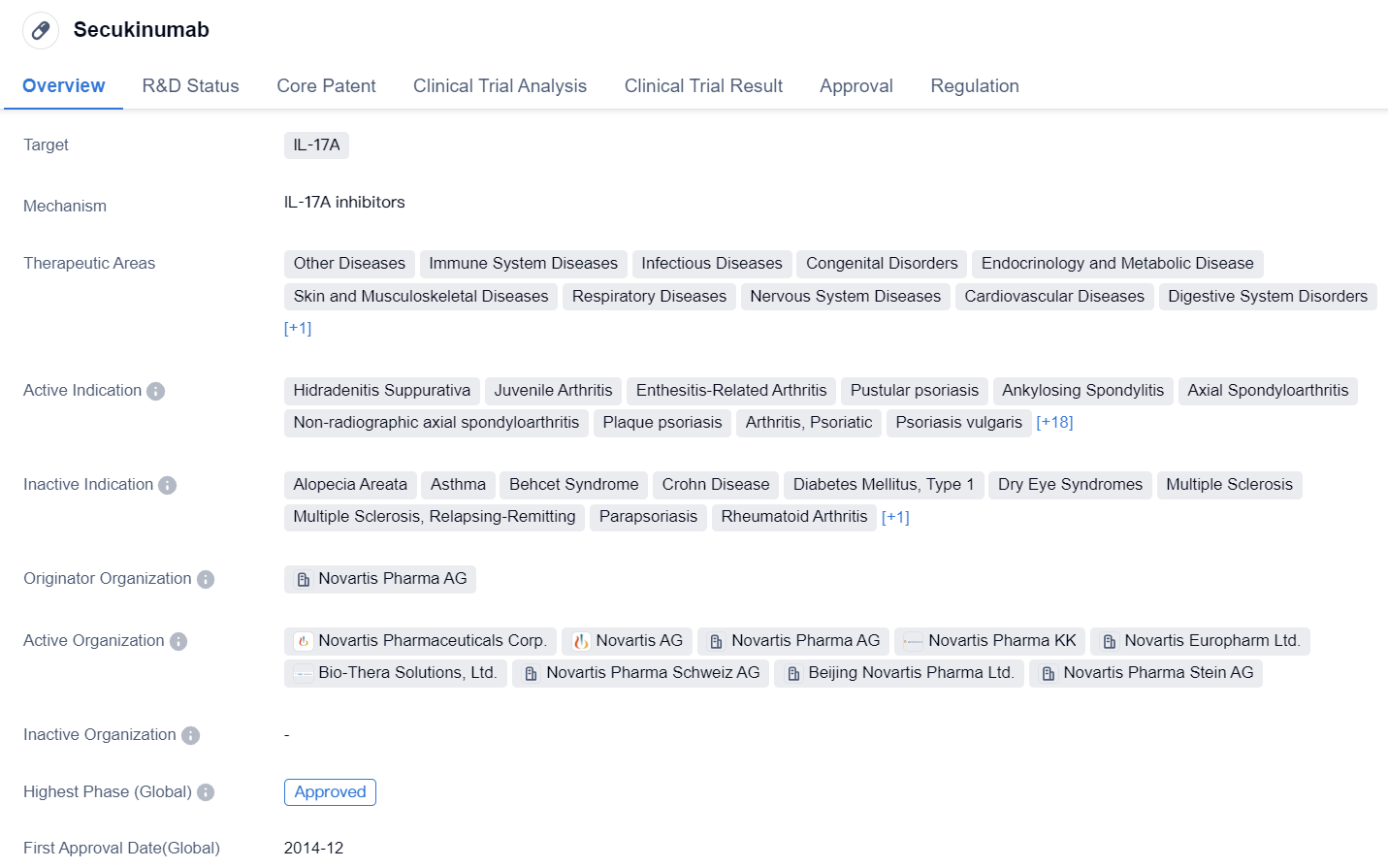
Secukinumab is a monoclonal antibody drug that targets IL-17A, a protein involved in various diseases related to the immune system, skin, musculoskeletal system, respiratory system, cardiovascular system, and more. It has been approved for multiple indications and has shown promising results in treating a wide range of conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has been approved for the treatment of Hidradenitis Suppurativa, Juvenile Arthritis, Enthesitis-Related Arthritis, Pustular psoriasis, Ankylosing Spondylitis, Axial Spondyloarthritis, Non-radiographic axial spondyloarthritis, Plaque psoriasis, Arthritis, Psoriatic, Psoriasis vulgaris, Rotator Cuff Tendinitis, Polymyalgia Rheumatica, Tendinopathy, Giant Cell Arteritis, Graves Ophthalmopathy, Lupus Nephritis, Nonalcoholic Steatohepatitis, Arthritis, Gastroenteritis, Psoriasis, SARS-CoV-2 acute respiratory disease, Lichen Planus, Lupus Erythematosus, Discoid, Necrobiosis Lipoidica, Tendon Injuries, Dermatitis, Atopic, Rotator Cuff Injuries, and Spondylarthritis.
Secukinumab was developed by Novartis Pharma AG and received its first approval in Japan in December 2014. It is classified as an overseas new drug urgently needed in clinical settings and has received priority review status. Additionally, it has been designated as an orphan drug, indicating its potential to treat rare diseases.
The highest phase of development for Secukinumab is approved, both globally and in China. This suggests that the drug has successfully completed clinical trials and has been deemed safe and effective for use in patients. The approval in multiple countries and for various indications highlights the broad therapeutic potential of Secukinumab.
In summary, Secukinumab is a monoclonal antibody drug that targets IL-17A and has been approved for the treatment of numerous diseases across different therapeutic areas. Developed by Novartis Pharma AG, it has shown significant promise in clinical trials and has received approvals in multiple countries. Its diverse range of indications and orphan drug designation further emphasize its potential in addressing unmet medical needs.
IL-17A/IL-17F Dual Inhibitor: Bimekizumab
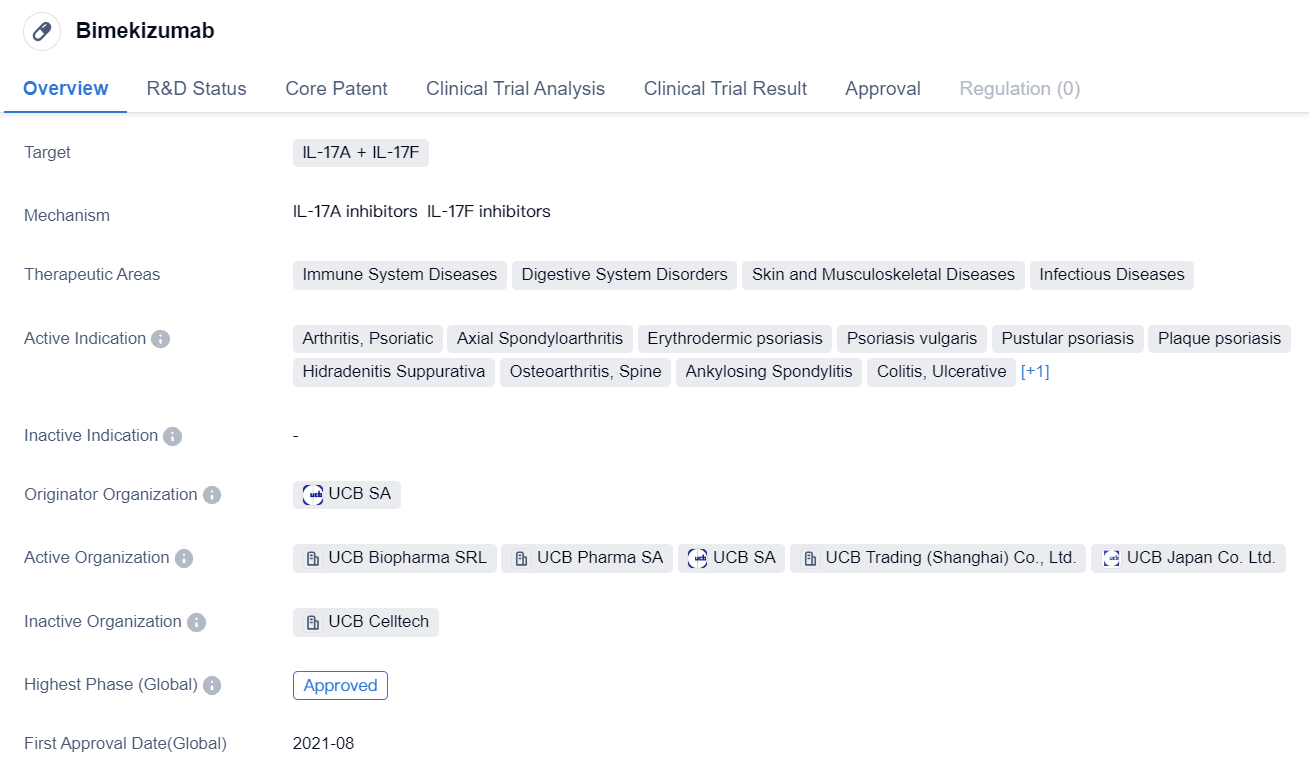
Bimekizumab is a monoclonal antibody drug that targets IL-17A and IL-17F. It falls under the therapeutic areas of immune system diseases, digestive system disorders, skin and musculoskeletal diseases, and infectious diseases. The drug has been approved for various indications, including arthritis (psoriatic, axial spondyloarthritis, rheumatoid arthritis), psoriasis (erythrodermic, vulgaris, pustular, plaque), hidradenitis suppurativa, osteoarthritis of the spine, ankylosing spondylitis, and ulcerative colitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Bimekizumab is UCB SA. It has reached the highest phase of approval globally, with its first approval granted in the European Union in August 2021. In China, the drug is currently in the NDA/BLA phase, indicating that it is seeking approval for marketing in the country.
Bimekizumab's mechanism of action involves targeting IL-17A and IL-17F, which are cytokines involved in the inflammatory response. By inhibiting these cytokines, the drug aims to reduce inflammation and alleviate symptoms associated with immune system diseases, digestive system disorders, and skin and musculoskeletal diseases.
The approval of Bimekizumab in the European Union signifies its efficacy and safety profile demonstrated in clinical trials. It offers a potential treatment option for patients suffering from various inflammatory conditions, including psoriasis, arthritis, and ulcerative colitis. The drug's approval in the European market also opens up opportunities for patients in other regions to potentially benefit from its therapeutic effects.
As Bimekizumab progresses through the NDA/BLA phase in China, it indicates the intention to expand its market reach and provide treatment options for patients in the country. The drug's approval in China would further contribute to addressing the unmet medical needs of individuals with immune system diseases, digestive system disorders, and skin and musculoskeletal diseases.
In summary, Bimekizumab is a monoclonal antibody drug that targets IL-17A and IL-17F. It has received approval in the European Union for various indications, including arthritis, psoriasis, and ulcerative colitis. With its potential to reduce inflammation and alleviate symptoms, Bimekizumab offers hope for patients suffering from immune system diseases, digestive system disorders, and skin and musculoskeletal diseases. Its ongoing NDA/BLA phase in China indicates the intention to expand its market presence and provide treatment options for patients in the country.