Advances in Clinical Research on Mcl-1 Inhibitors
Mcl-1, also known as myeloid cell leukemia-1, is a protein that plays a crucial role in regulating cell survival and apoptosis (programmed cell death) in the human body. It belongs to the Bcl-2 family of proteins, which are involved in maintaining the balance between cell survival and death. Mcl-1 is primarily found in various tissues, including the immune system, and is particularly important for the survival of immune cells. Dysregulation of Mcl-1 expression or function has been implicated in various diseases, including cancer, where it can promote tumor cell survival and resistance to chemotherapy. Therefore, targeting Mcl-1 has emerged as a potential therapeutic strategy in the pharmaceutical industry for the treatment of cancer and other diseases.
Mcl-1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 20 Sep 2023, there are a total of 32 Mcl-1 drugs worldwide, from 49 organizations, covering 25 indications, and conducting 18 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.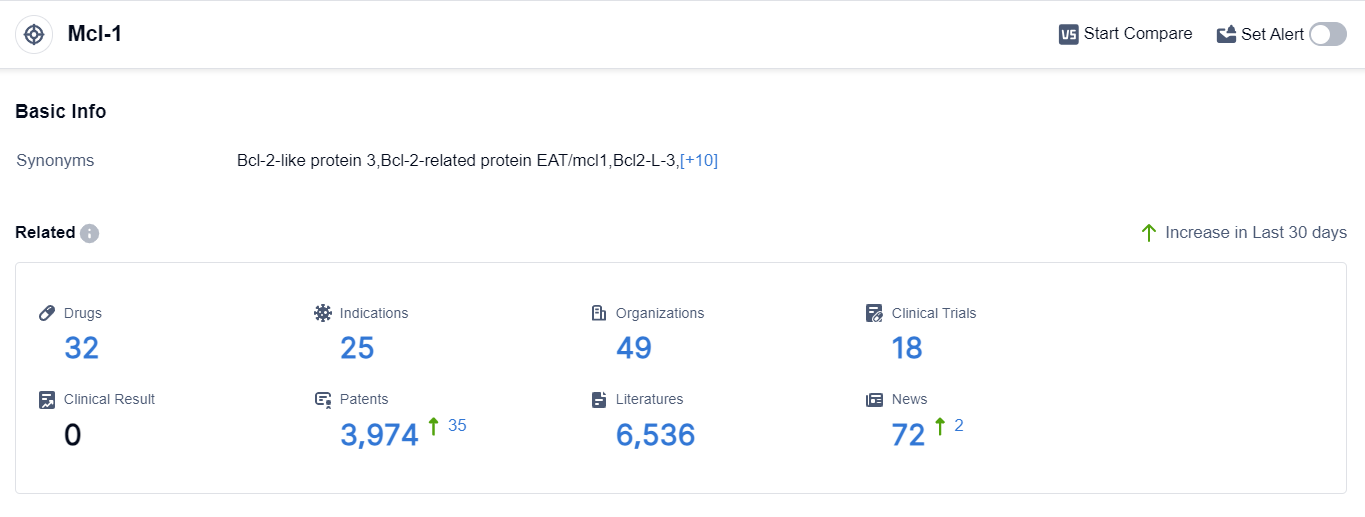
Based on the analysis of the provided data, the current competitive landscape of target Mcl-1 in the pharmaceutical industry is characterized by the involvement of multiple companies, with Novartis AG, Les Laboratoires Servier SAS, Ligand Pharmaceuticals, Inc., and AstraZeneca PLC being the key players. These companies have shown significant R&D progress in the development of drugs targeting Mcl-1.
Drugs targeting Mcl-1 have been approved for various indications, including Acute Myeloid Leukemia, Breast Cancer, Hematologic Neoplasms, Multiple Myeloma, Non-Hodgkin Lymphoma, Solid Tumors, Myelodysplastic Syndromes, Lung Cancer, Sarcoma, and others.
Small molecule drugs, Unknown drugs, Biological products, PROTACs, Chemical drugs, Small interfering RNA, and Monoclonal antibodies are the drug types progressing most rapidly under the current target Mcl-1.
The United States, European Union, and China are the countries/locations with the highest development progress under the current target. China has shown progress in Phase 1 and Preclinical phases.
Overall, the target Mcl-1 presents a competitive landscape with multiple companies and a diverse range of indications and drug types. Further research and development in these areas are expected to drive the future development of drugs targeting Mcl-1 in the pharmaceutical industry.
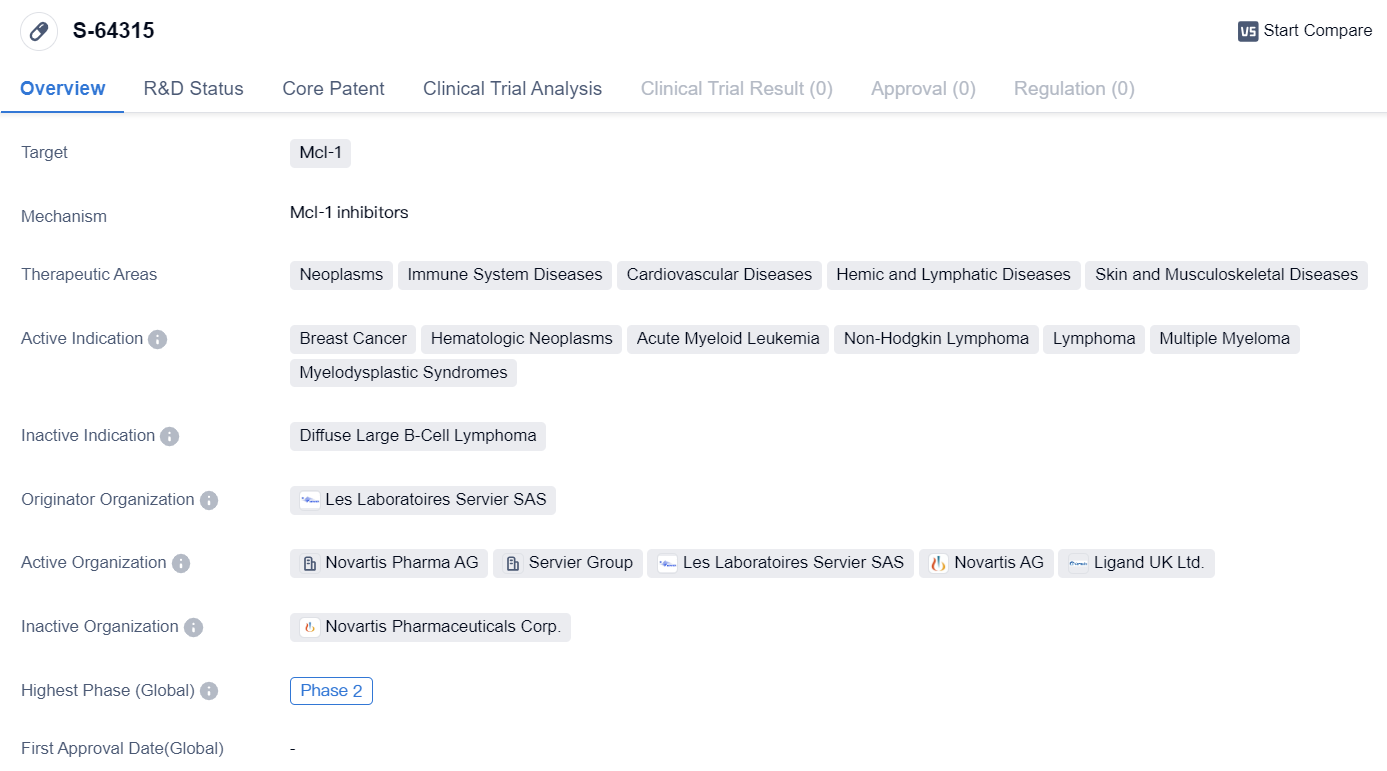
Mcl-1 inhibitors entering Phase II temporary research: S-64315
S-64315 is a small molecule drug that targets Mcl-1, a protein involved in cell survival and apoptosis regulation. This drug is being developed by Les Laboratoires Servier SAS, a pharmaceutical company known for its research and development in various therapeutic areas.
The therapeutic areas that S-64315 aims to address include neoplasms (abnormal growth of cells), immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. These areas encompass a wide range of medical conditions, highlighting the potential versatility of this drug.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of active indications, S-64315 is being investigated for its efficacy in treating breast cancer, hematologic neoplasms (cancers affecting blood cells), acute myeloid leukemia (a type of blood cancer), non-Hodgkin lymphoma, lymphoma, multiple myeloma (a cancer of plasma cells), and myelodysplastic syndromes (a group of disorders characterized by abnormal blood cell production).
Currently, S-64315 has reached Phase 2 of clinical development. This phase involves testing the drug on a larger group of patients to evaluate its safety, dosage, and effectiveness. Phase 2 trials are crucial in determining whether a drug shows enough promise to progress to the next phase of development.
Based on the available information, S-64315 appears to be a promising drug candidate with a broad potential for treating various diseases. Its specific targeting of Mcl-1 suggests a focus on inhibiting cell survival mechanisms, which could be particularly relevant in cancer treatment. However, without further details or clinical trial results, it is difficult to assess the drug's potential efficacy and safety profile.
In conclusion, S-64315 is a small molecule drug developed by Les Laboratoires Servier SAS, targeting Mcl-1. It aims to address a wide range of therapeutic areas, including neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The drug is currently in Phase 2 of clinical development and is being investigated for its potential in treating breast cancer, hematologic neoplasms, acute myeloid leukemia, non-Hodgkin lymphoma, lymphoma, multiple myeloma, and myelodysplastic syndromes. Further research and clinical trials are needed to determine the drug's effectiveness and safety.
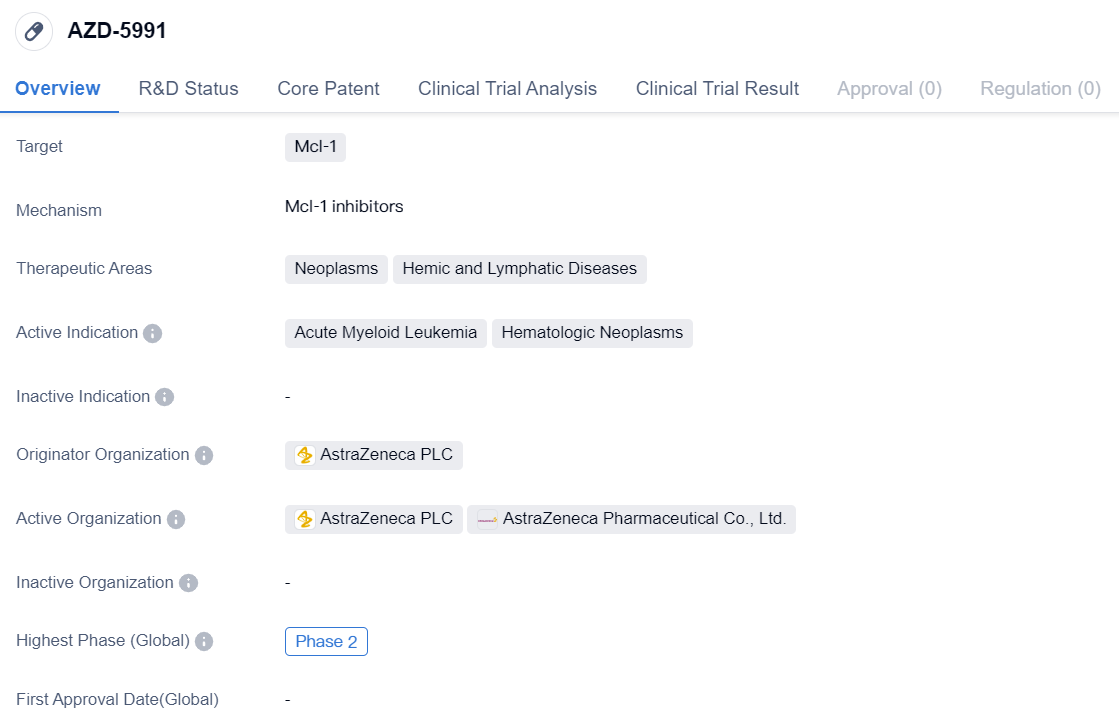
AZD-5991
AZD-5991 is a small molecule drug developed by AstraZeneca PLC, a leading pharmaceutical company. It is designed to target Mcl-1, a protein involved in the regulation of cell survival and apoptosis. The drug is being developed for the treatment of neoplasms, which are abnormal growths of tissue, and hemic and lymphatic diseases.
The active indications for AZD-5991 include acute myeloid leukemia and hematologic neoplasms. Acute myeloid leukemia is a type of cancer that affects the bone marrow and blood, leading to the production of abnormal white blood cells. Hematologic neoplasms refer to a group of cancers that affect the blood, bone marrow, and lymphatic system.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Currently, AZD-5991 is in Phase 2 of clinical development. Clinical trials are conducted in different phases to evaluate the safety and efficacy of a drug in humans. Phase 2 trials involve a larger number of participants and aim to gather more data on the drug's effectiveness and side effects.
The development of AZD-5991 as a small molecule drug indicates that it is a chemical compound with a relatively low molecular weight. Small molecule drugs are commonly used in pharmaceutical research and development due to their ability to interact with specific targets in the body.
AstraZeneca PLC, the originator organization of AZD-5991, is a multinational pharmaceutical company known for its expertise in the development of innovative drugs. The company has a strong presence in the biopharmaceutical industry and is committed to advancing healthcare through scientific research and development.
In summary, AZD-5991 is a small molecule drug developed by AstraZeneca PLC, targeting Mcl-1. It is being developed for the treatment of neoplasms and hemic and lymphatic diseases, specifically acute myeloid leukemia and hematologic neoplasms. Currently in Phase 2 of clinical development, AZD-5991 shows promise as a potential therapeutic option for patients with these conditions.