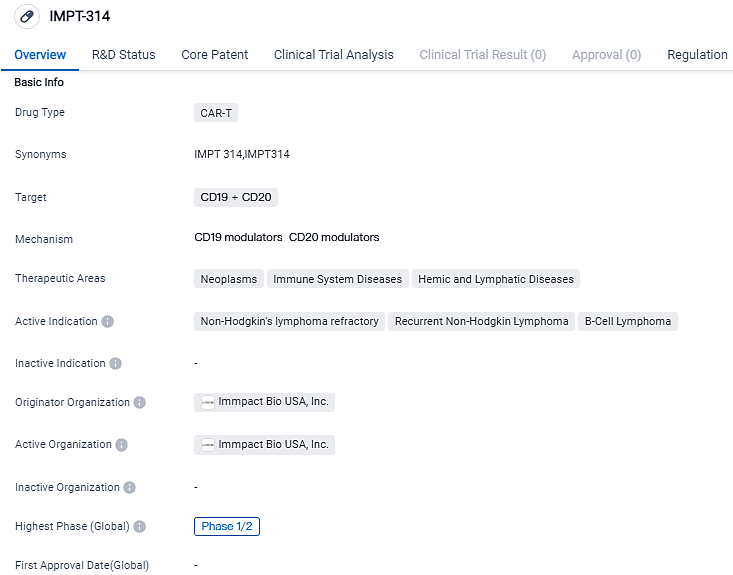
The first participant in ImmPACT Bio's Phase 1/2 study has received treatment with IMPT-314, aimed at combatting aggressive B-cell Lymphoma
ImmPACT Bio USA, Inc., a firm at the clinical phase that is working on the creation of revolutionary logic-gate-based CAR T-cell therapies aimed at battling cancer and autoimmune diseases, has publicized that the initial patient has received treatment in the Phase 1/2 trial of IMPT-314. IMPT-314 is a dual-specific CD19/CD20 CAR T-cell therapy designed for the therapy of recurrent or stubborn aggressive B-cell lymphoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"It gives us great satisfaction to announce that we administered the initial treatment of IMPT-314, and to contribute to the clinical evidence from the current investigator-led exploration of this dual-targeted CD19/CD20 CAR T-cell therapy. Our optimistic standpoint is that IMPT-314 can potentially enhance tolerability and durability of responses in patients suffering from B-cell lymphomas, putting it in the best-in-class category," stated Dr. Jonathan Benjamin, Ph.D., ImmPACT Bio's Chief Medical Officer.
Dr. Sarah Larson, who is the principle investigator and an associate clinical professor of medicine, further added, "Despite CD19 CAR T-cell therapies revolutionizing lymphoma treatment, durability constraints persist due to treatment resistance, which is a result of selective pressure against the target antigen leading to a subsequent loss of expression. We eagerly to delineating the differentiated therapeutic potential of this bispecific approach through data from ImmPACT Bio's Phase 1/2 IMPT-314 trial."
ImmPACT Bio's Phase 1/2 trial is a multi-center, open-label clinical investigation implemented across several centers and will evaluate the safety and effectiveness of IMPT-314 on those diagnosed with R/R aggressive B-cell lymphoma. Both CAR T-experienced and CAR T-naïve patients will be included in Phase 1 of this dose-finding study. The U.S. FDA has given a Fast Track Designation to IMPT-314 for treatment of R/R aggressive B-cell lymphoma.
B-cell lymphomas, a type of blood cancer that affects the immune system's B cells, often recur and necessitate several rounds of therapy. Despite the availability of multiple approved treatments, such as CD19-targeted CAR T-cell therapies, there is still a significant gap in the provision of safe and highly effective treatments for B-cell lymphomas.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
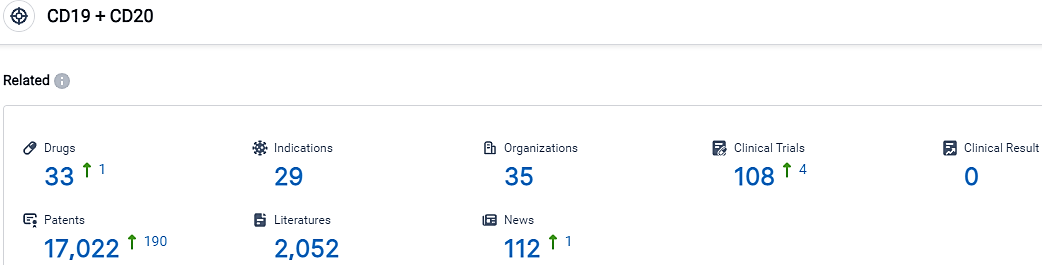
According to the data provided by the Synapse Database, As of September 25, 2023, there are 33 investigational drugs for the CD19 and CD20 target, including 29 indications,35 R&D institutions involved, with related clinical trials reaching 108,and as many as 17022 patents.
IMPT-314 is a CD19/CD20-targeting CAR T-cell therapy that utilizes a potent bispecific CAR and a 4-1BB costimulatory domain. The drug is currently in Phase 1/2 of development and has been granted Fast Track status, indicating its potential to address unmet medical needs in these therapeutic areas. IMPT-314 has received Fast Track Designation from the U.S. FDA for the treatment of R/R aggressive B-cell lymphoma.