Sirius Therapeutics Launches Phase One Study for Innovative Anticoagulant to Combat Thromboembolic Conditions
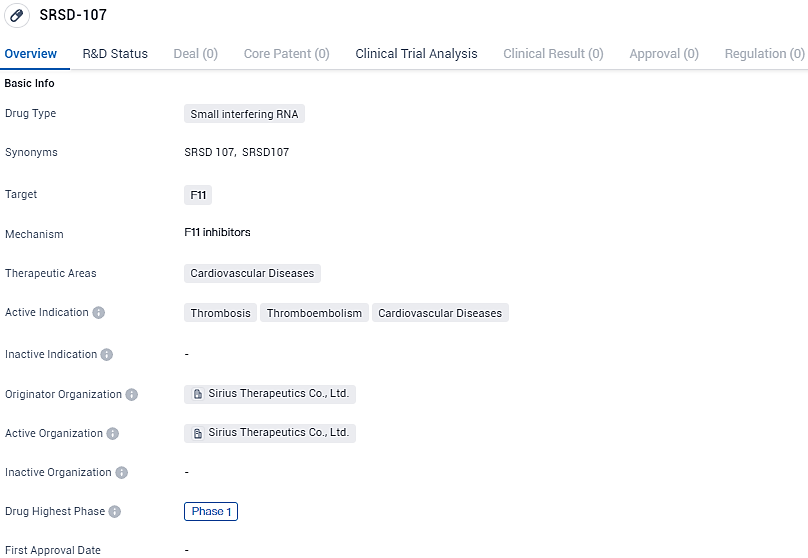
Sirius Therapeutics disclosed that it initiated dosing with its innovative siRNA therapeutic, SRSD107, targeting the coagulation Factor XI (FXI) in the inaugural participant of a Phase 1 clinical trial conducted in Australia. This trial is focused on investigating SRSD107 for its potential in both preventing and managing thromboembolic conditions.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The biopharmaceutical enterprise has introduced SRSD107 as their second promising agent within the collection of innovative siRNA formulations aimed at tackling heart-related ailments. The firm has initiated a clinical study for SRSD101 within the Chinese territory to address abnormal lipid levels, subsequent to receiving the nod from the regulatory body, the China National Medical Products Administration.
Dr. Qunsheng Ji, at the helm of Sirius Therapeutics, shared insights on the trial which leverages data from in vivo examinations showing an almost complete suppression of FXI concentrations that persisted up to half a year, following a one-time administration via the subcutaneous route, notably without any hemorrhagic complications. Dr. Ji underlined that the ongoing trial will scrutinize the distinctive treatment capacity of SRSD107 for these widely-encountered health concerns on a worldwide scale.
The initial phase trial of SRSD107, which is underway with the participation of healthy individuals in Australia, aims to discern the compound's safety profile, its ease of tolerance in humans, its pharmacokinetic attributes, and its pharmacodynamic influences, assessing both single-rising doses and escalating doses over time.
Cardiovascular events, like heart attacks, strokes rooted in an ischemic cause, and clotting in the veins, commonly stem from thrombosis. A global mortality research published by The Lancet reveals these thromboembolic complications are believed to lead to one out of every four deaths globally.
Sirius Therapeutics has developed the unique double-strand siRNA molecule, SRSD107, with a precise mechanism aimed at the gene expression of coagulation factor XI, diminishing the synthesis of the FXI protein which in turn inhibits the intrinsic pathway of blood coagulation and fosters anticoagulation and anti-thrombotic benefits. The design of SRSD107 aspires to extend its dosing interval, potentially up to once or twice annually.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
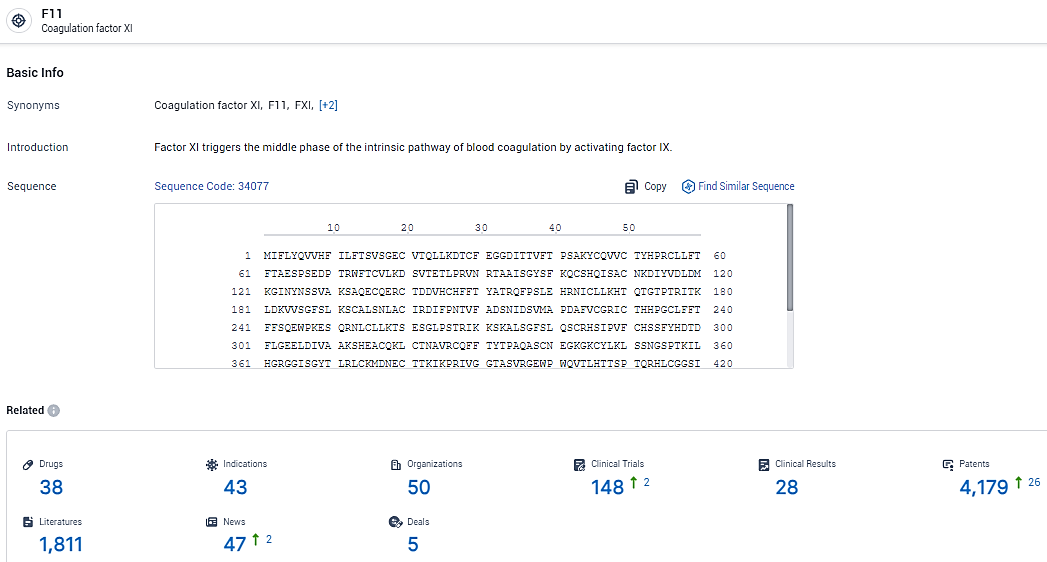
According to the data provided by the Synapse Database, As of February 7, 2024, there are 38 investigational drugs for the F11 target, including 43 indications, 50 R&D institutions involved, with related clinical trials reaching 148, and as many as 4179 patents.
SRSD-107 targets the F11 gene and is intended for the treatment of cardiovascular diseases, specifically thrombosis, thromboembolism, and related conditions. While it has reached Phase 1 globally, it is currently at the IND application stage in China. Further research and clinical trials will be necessary to determine the drug's efficacy and safety in treating these cardiovascular disorders.