Tumor Killers: STAT3 inhibitors
Signal transducer and activator of transcription (STAT) is a class of proteins with dual functions of cytoplasmic signal transduction and nuclear transcription activation, widely found in mammalian cells, mediating a variety of intracellular signal pathways. Although STAT family members are highly homologous, their functions are vastly diverse.
STAT3 is a signaling molecule expressed in various tissues. STAT3 can be activated by various factors, including cytokines, growth factors, neuroendocrine factors, ischemic hypoxic stimuli, etc. As a functionally diversified transcription factor, STAT3 can interact with a large number of signal molecules and forms various intra- and extracellular signal pathways.
STAT3 is involved in a variety of biological processes, including cell proliferation, survival, differentiation and angiogenesis. In normal cells, STAT3 is transiently activated by phosphorylation to transmit the transcription signals of cytokine and growth factor receptors on the plasma membrane to the nucleus. Studies have shown that persistently activated STAT3 is essential for various cancers, such as breast cancer and colorectal cancer, making STAT3 an ideal drug target. However, STAT3 becomes excessively activated in most human cancers, usually associated with poor clinical prognosis.
Through signal transduction and transcription activation, STAT3 participates in complex and varied life activities in biological bodies and is a current research hotspot. The research on STAT3 in the field of tumors has already achieved staged results. As the convergence point of multiple tumor pathways, STAT3 is persistently highly expressed in tumor cells and can promote tumor cell proliferation, resist cell apoptosis, mediate oncogenic inflammation, and inhibit anti-tumor immunity, etc. STAT3 has become an important molecular target for tumor treatment to a certain extent!
STAT3 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 25 Sep 2023, there are a total of 85 STAT3 drugs worldwide, from 92 organizations, covering 103 indications, and conducting 161 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.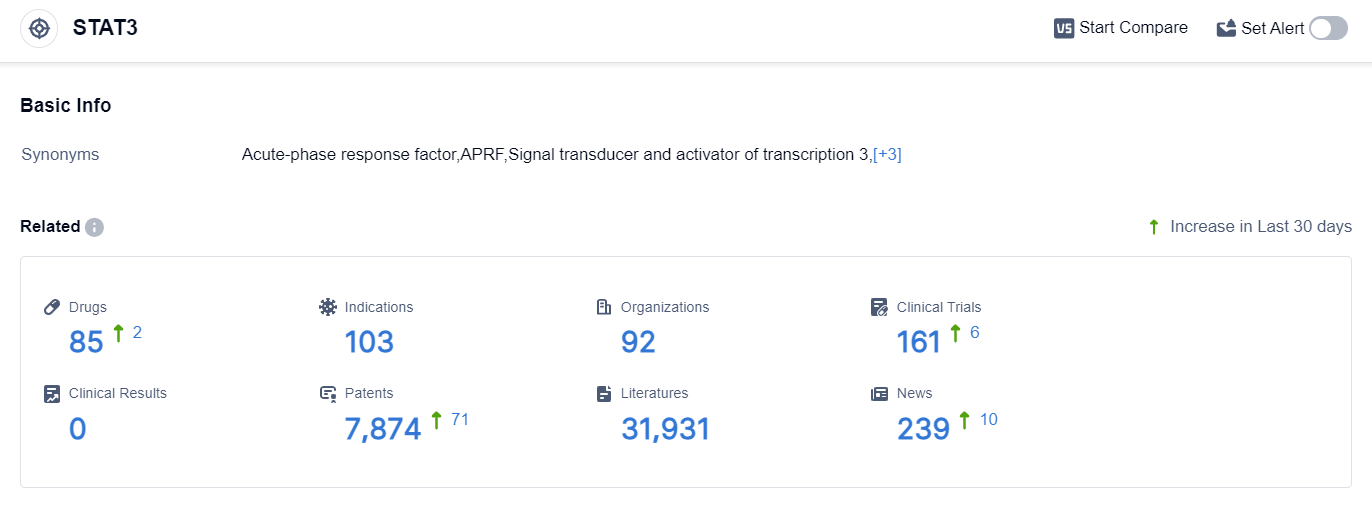
The analysis of the current competitive landscape and future development of target STAT3 reveals that multiple companies are actively involved in the research and development of drugs targeting this specific target. The highest stage of development is the approved stage, with companies like Verta, Inc., Beijing Union Pharmaceutical Factory, and Sumitomo Chemical Co., Ltd. leading the way.
Neoplasms and hepatocellular carcinoma are among the indications with approved drugs. Small molecule drugs are progressing rapidly, followed by antisense oligonucleotides, AAV-based gene therapy, and PROTACs.
China, the United States, and various countries in the European Union are developing rapidly under the target STAT3. Further analysis is required to understand the competition around innovative drugs and the research and development institutions involved in biosimilars. Overall, the target STAT3 shows promising potential for the development of new drugs in the pharmaceutical industry.
STAT3 inhibitors entering into Phase III clinical trials: Napabucasin
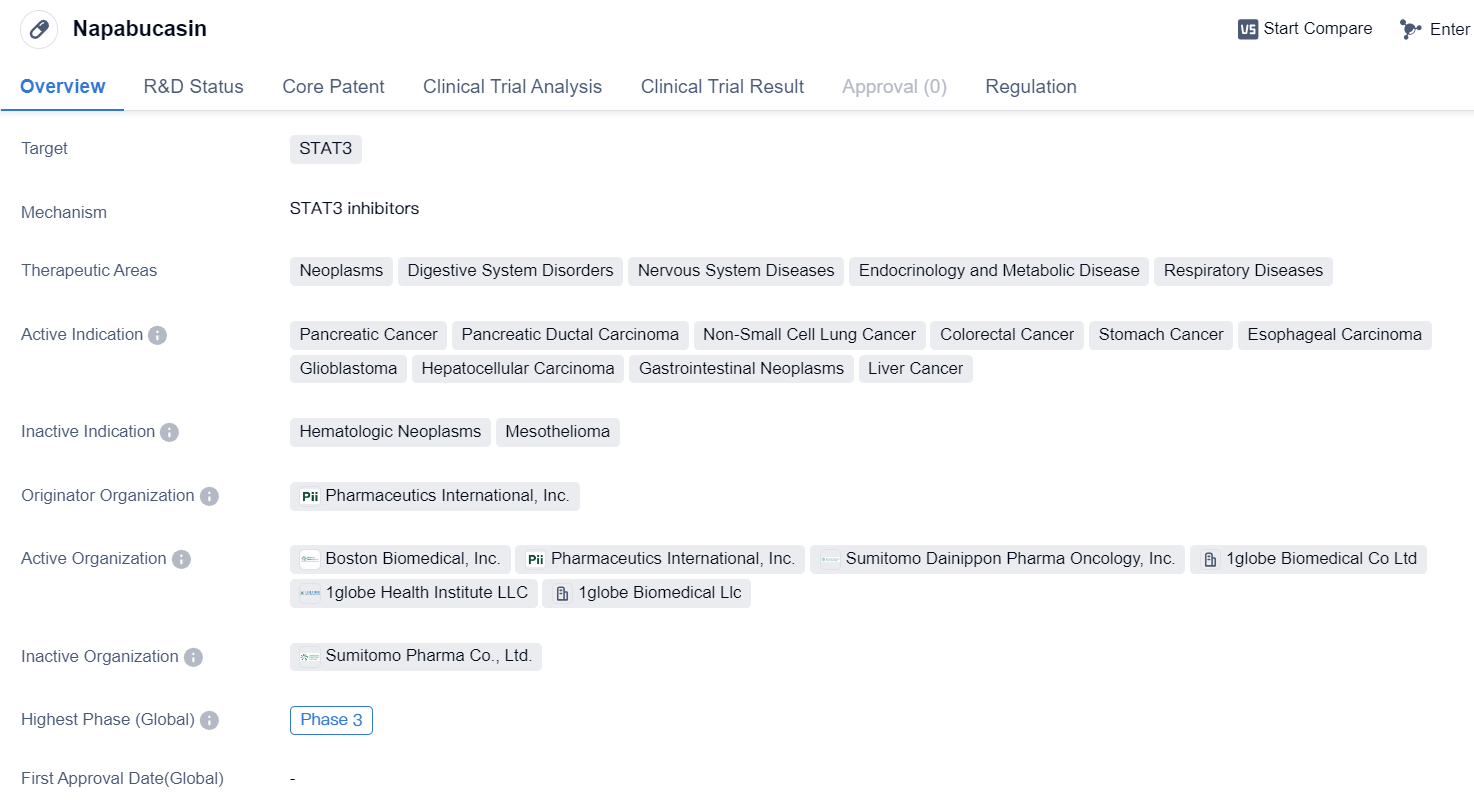
Napabucasinis a small molecule drug that targets STAT3, a protein involved in various cellular processes, including cell growth and survival. It has shown potential therapeutic benefits in several therapeutic areas, including neoplasms, digestive system disorders, nervous system diseases, endocrinology and metabolic diseases, and respiratory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of specific indications, Napabucasin has demonstrated activity against pancreatic cancer, pancreatic ductal carcinoma, non-small cell lung cancer, colorectal cancer, stomach cancer, esophageal carcinoma, glioblastoma, hepatocellular carcinoma, gastrointestinal neoplasms, and liver cancer. These indications cover a wide range of cancers affecting different organs and systems in the body.
The drug is developed by Pharmaceutics International, Inc., an originator organization in the pharmaceutical industry. It has reached Phase 3 of clinical development globally and in China. Phase 3 trials are the final stage of clinical development before seeking regulatory approval.
Napabucasin has been designated as an orphan drug, indicating that it is intended to treat rare diseases or conditions. This regulatory status provides certain incentives and benefits to the drug's developer, such as market exclusivity and financial incentives, to encourage the development of treatments for rare diseases.
Based on the available information, Napabucasin shows promise as a potential treatment for various types of cancer and other diseases. However, further clinical trials and regulatory approvals are necessary to establish its safety and efficacy. The drug's specific mechanism of action, potential side effects, and other relevant details are not provided in the given information. Therefore, a comprehensive analysis of the drug's potential and its competitive landscape would require additional data and research.
STAT3 inhibitors entering into Phase II clinical trials: Danvatirsen
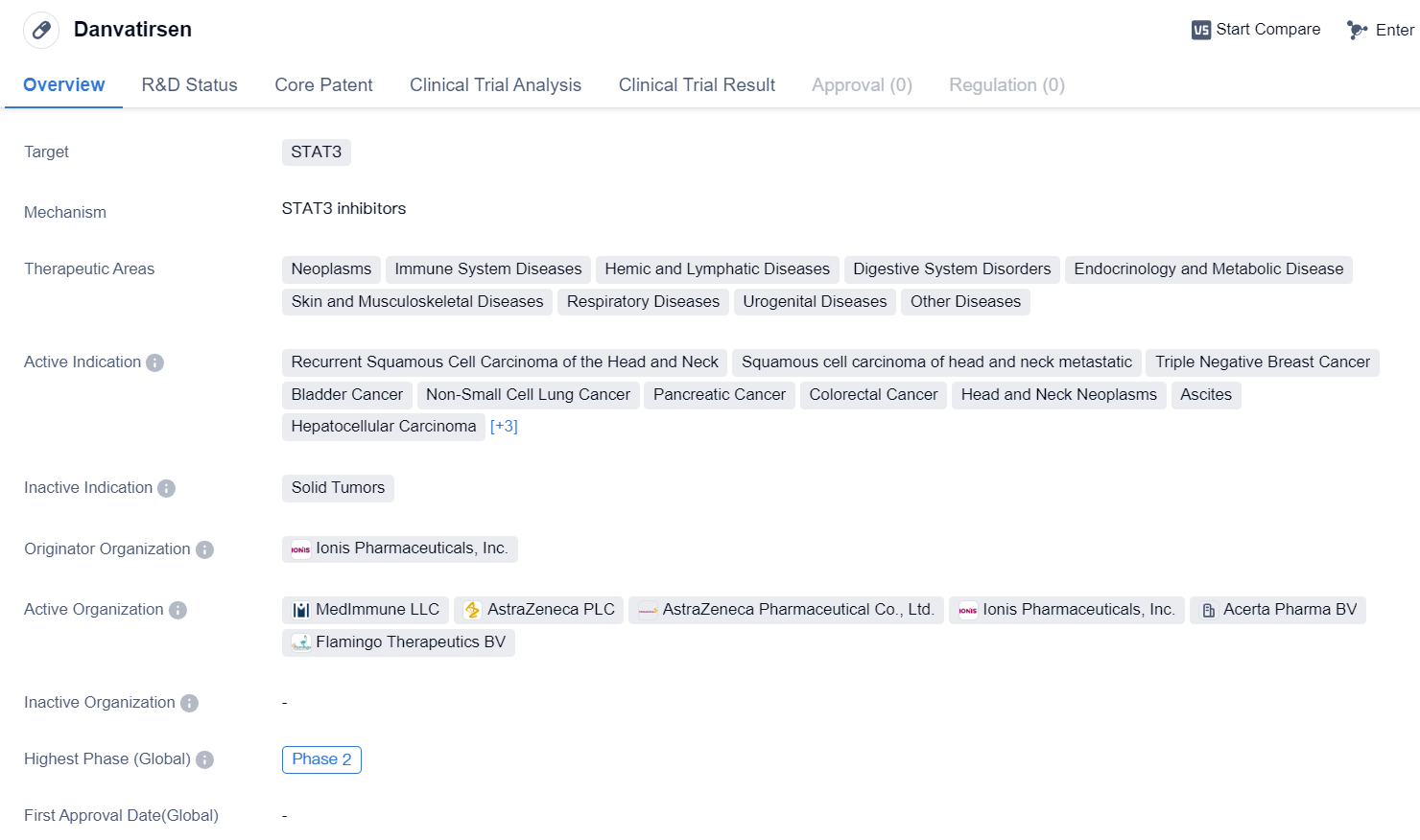
Danvatirsen is an antisense oligonucleotide drug that targets STAT3, a protein involved in various cellular processes, including cell growth and survival. It is being developed by Ionis Pharmaceuticals, Inc. and has reached Phase 2 of clinical trials, both globally and in China.
The therapeutic areas targeted by Danvatirsen include neoplasms (abnormal growth of cells), immune system diseases, hemic and lymphatic diseases, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, and other diseases. This broad range of therapeutic areas suggests that Danvatirsen has the potential to be used in the treatment of various conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug's active indications include recurrent squamous cell carcinoma of the head and neck, squamous cell carcinoma of head and neck metastatic, triple-negative breast cancer, bladder cancer, non-small cell lung cancer, pancreatic cancer, colorectal cancer, head and neck neoplasms, ascites (abnormal accumulation of fluid in the abdomen), hepatocellular carcinoma (liver cancer), non-Hodgkin lymphoma, and diffuse large B-cell lymphoma.
As an antisense oligonucleotide, Danvatirsen works by binding to the messenger RNA (mRNA) molecules that carry the instructions for producing STAT3 protein. By binding to these mRNA molecules, Danvatirsen prevents the production of STAT3 protein, thereby inhibiting its activity. This approach offers a targeted and specific way to interfere with the function of STAT3, which is known to play a role in the development and progression of various cancers.
The fact that Danvatirsen has reached Phase 2 of clinical trials indicates that it has shown promising results in earlier stages of testing, such as Phase 1 trials. Phase 2 trials involve a larger number of participants and aim to further evaluate the drug's safety and effectiveness. The inclusion of China in the highest phase of development suggests that Ionis Pharmaceuticals, Inc. recognizes the potential market and demand for Danvatirsen in this country.
In summary, Danvatirsen is an antisense oligonucleotide drug developed by Ionis Pharmaceuticals, Inc. that targets STAT3. It has shown potential in a wide range of therapeutic areas, particularly in the treatment of various cancers. With its current highest phase being Phase 2, Danvatirsen is undergoing further evaluation to determine its safety and effectiveness.