YS Biopharma Initiates Patient Registration for Key Phase 3 Study on PIKA Rabies Vaccine
As a worldwide leader in the biopharmaceutical industry, YS Biopharma Co., Ltd., pledged to discovery, develop, produce, and supply innovative vaccines and therapeutic biologics for infectious illnesses and cancer. News has been released that they have achieved a milestone by recruiting the initial participant for their Phase 3 clinical study for the firm's PIKA Rabies Vaccine.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
With an aim of evaluating its safety, immunogenicity, and batch-by-batch reliability, the test of the PIKA Rabies Vaccine is planning to encapsulate roughly 4,500 participants. Rabies is a viral illness defined by nearly 100% fatality rate when symptomatic. This virus is approximated to cause 59,000 human deaths each year in over 150 nations, largely in Asia and Africa. Transmissions from dog bites account for more than 95% of rabies-related deaths, with 40% of fatalities being children under 15 years old.
Even though rabies usually leads to death if not treated, it's possible to prevent the fatality by applying post-exposure prophylaxis following possible exposure. The PIKA Rabies Vaccine, employing YS Biopharma's unique PIKA adjuvant technology, aims to induce a stronger immune reaction in a shorter duration as compared to existing rabies vaccines. Earlier Phase 1 and Phase 2 trials of this vaccine have proved its safety and significant immunogenicity. They've shown that it triggers a noticeable immune response in as short as a week.
This demonstrates that PIKA Rabies Vaccine holds the potential to offer superior expedited protection, in line with WHO's objective of achieving a one-week rabies vaccine regimen to supersede the usual three-four week regimens.
The Principal Researcher, Dr. Muhammad Ahmad from Central Park Teaching Hospital, Lahore, Pakistan, where the first participant has been registered, remarked, "We appreciate the collective efforts of premier institutions and clinical researchers worldwide who will be generating clinical and scientific evidence on the PIKA Rabies Vaccine in significant populations. We have hope that these findings will direct the future of vaccine interventions and contribute to addressing a significant global public health concern."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
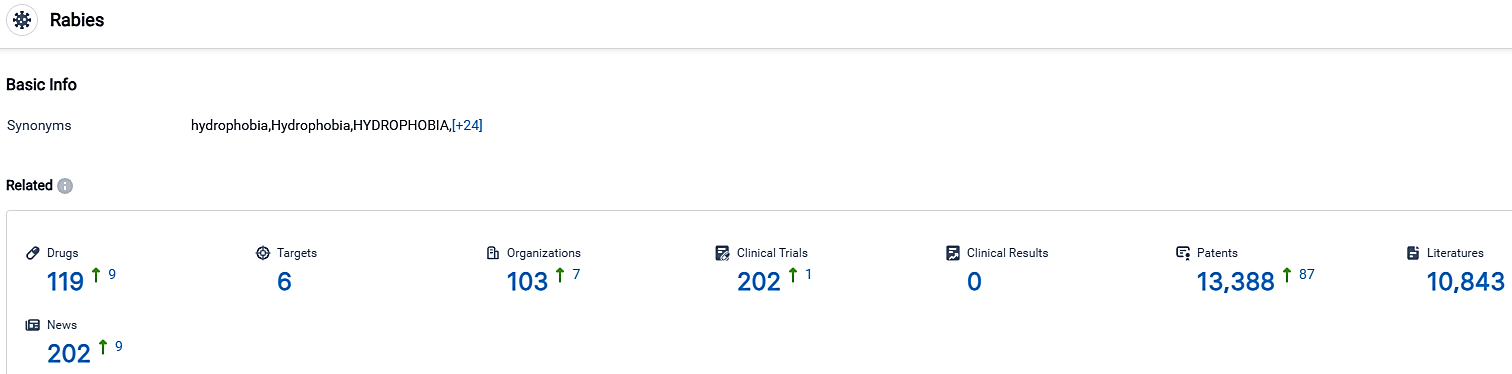
According to the data provided by the Synapse Database, As of October 2, 2023, there are 119 investigational drugs for the Rabies, including 6 targets, 103 R&D institutions involved, with related clinical trials reaching 202,and as many as 13388 patents.
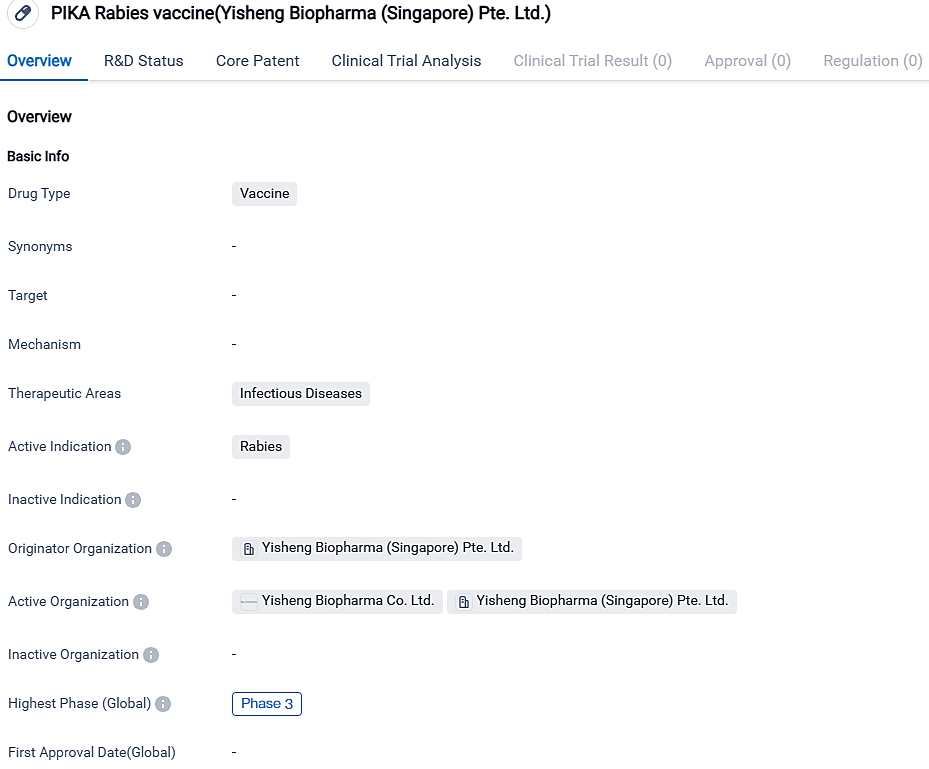
Yisheng Biopharma Pte. Ltd. is the creator of the PIKA Rabies vaccine, which is a medicine made to halt the transmission of the infectious condition known as rabies. It is at present, undergoing Phase 3 of clinical testing worldwide and domestically in China. This vaccine offers substantial promise in battling this lethal disease and diminishing its worldwide prevalence.