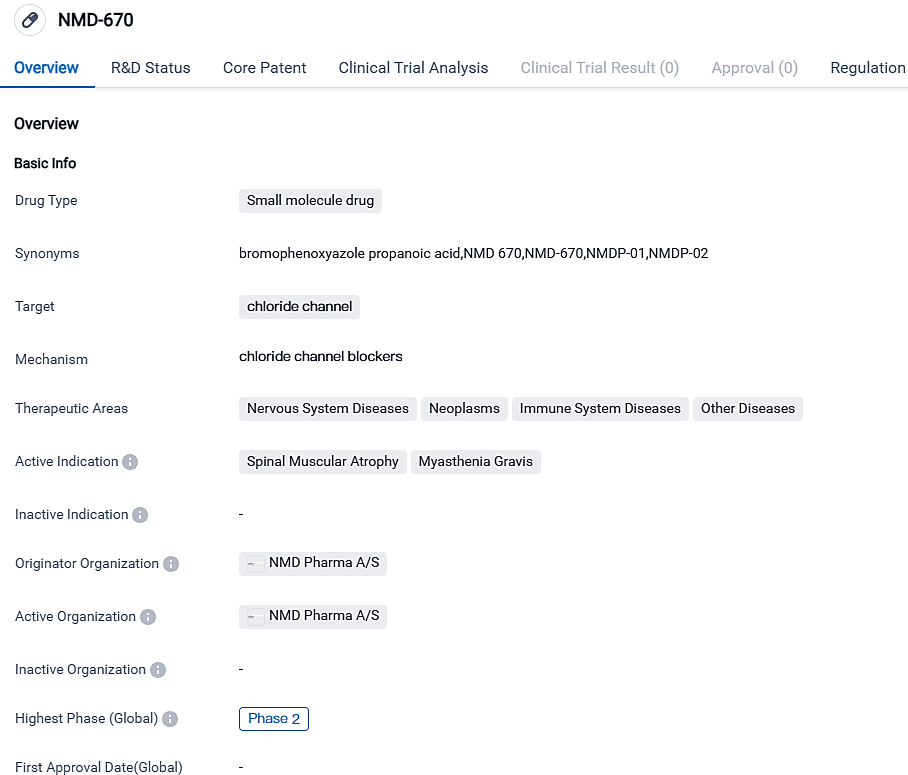
NMD Pharma has launched a second-phase clinical trial for NMD670 in the treatment of Spinal Muscular Atrophyc
NMD Pharma A/S, a biotech firm in the clinical stage specializing in the development of pioneering ClC-1 blockers that aim at muscle function in neuromuscular diseases, has revealed that the initial patient has received their first dose in a Phase II clinical study of the ClC-1 inhibitor NMD670 in individuals affected by spinal muscular atrophy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The patient received treatment under the guidance of Prof. dr. Kristl Claeys, MD, PhD at the Kliniekhoofd Neurologie, Neuromusculaire Ziekten, Laboratory for Muscle Diseases and Neuropathies at UZ Leuven University Hospital in Belgium.
The clinical trial is in the Phase II stage and is a double-blind, placebo-controlled, randomized, 2-way crossover investigation aimed at assessing the efficiency, safety, and acceptability of administering NMD670 orally two times a day for three weeks in mobile adult patients suffering from SMA Type 3. The study incorporates multiple research centers around North America and Europe, making it an international endeavor.
"SMA is a rare neuromuscular disease that results in significant muscle weakness and fatigue having an extensive impact on the patient's and their family's quality of life," stated Jorge A. Quiroz, EVP and Head Medical Officer NMD Pharma. "The drug NMD670 has already demonstrated safe and quantifiable positive outcomes from a mechanism trial conducted on myasthenia gravis patients. We strongly believe that it has the potential to have similar beneficial effects in treating patients affected by SMA."
Adding to the conversation, Thomas Holm Pedersen, Chief Executive Officer of NMD Pharma, said: "While we've seen significant breakthroughs in therapies available to treat SMA, patients are still experiencing muscle weakness and limited mobility. We're thrilled to announce the commencement of treatment for the first participant in our Phase II trial. It's a huge landmark moment for our company as we progress and widen our range of potential treatment options."
"We eagerly anticipate the start of the Phase II trial looking at a fresh approach to dealing with SMA. Building on the progress of our current genetically targeted therapies that aim to modify the disease, NMD670 may play a role in restoring muscle strength and functionality by targeting the neuromuscular junction, which is known to be compromised in SMA," said Kenneth Hobby, President, of Cure SMA.
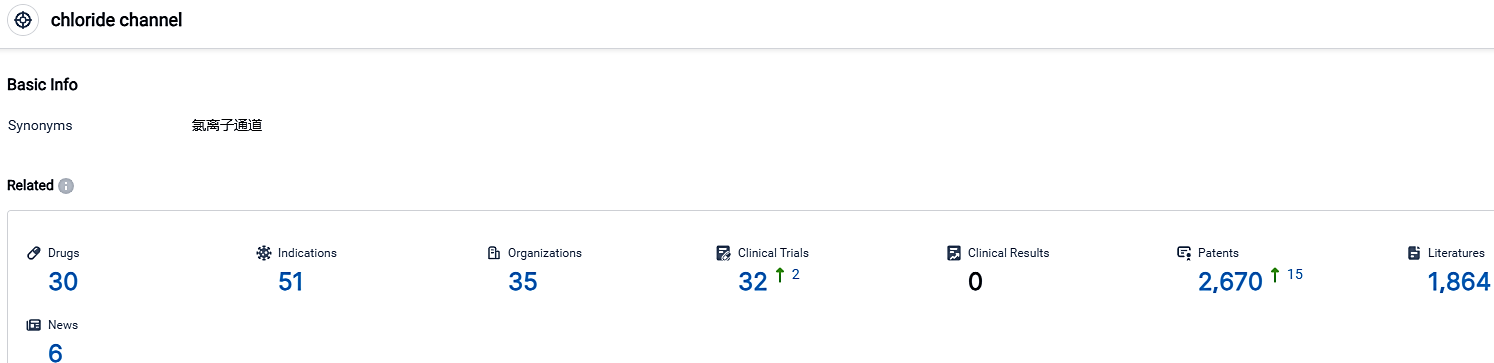
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 30, 2023, there are 30 investigational drugs for the chloride channel target, including 51 indications,35 R&D institutions involved, with related clinical trials reaching 32,and as many as 2670 patents.
NMD670 is a pioneer small molecule oppressor of the muscle-exclusive chloride ion passage, also known as the ClC-1 ion channel. Pre-clinical trials by NMD Pharma have verified that halting ClC-1 has the potential to improve neuromuscular communication and overall skeletal muscle operation across various animal neuromuscular disease models. Based on both these clinical trials and preclinical evidence, it is anticipated that this innovative procedure could prove advantageous in treating individuals affected by SMA.